WuXi Biologics
Offering End-to-End Solutions
The Evolution of Antibody-Drug Conjugate (ADC) Linker Design: A Snapshot of Key Innovations
The Evolution of Antibody-Drug Conjugate (ADC) Linker Design: A Snapshot of Key Innovations
Since Paul Ehrlich’s visionary “Magic Bullet” concept over a century ago, the pursuit of selectively delivering potent cytotoxins to tumor cells has been a cornerstone of oncology research. Antibody-Drug Conjugates (ADCs) represent the most advanced realization of this paradigm.
ADCs are intricate “targeted delivery systems” comprising a monoclonal antibody (which recognizes specific tumor-associated antigens), a cytotoxic payload (designed to kill tumor cells efficiently), and a linker that connects the antibody to the payload. The antibody can be viewed as the “navigation system,” the payload as the “warhead.” Central to this triad is the linker—a molecular scaffold that not only tethers the payload to the antibody but also governs the ADC’s pharmacokinetics, stability, and intracellular drug release kinetics. As such, linker design is a critical determinant of the therapeutic index and clinical success of ADCs.
Key Components and Classification of ADC Linkers
The typical structure of an ADC linker includes: (1) a bioconjugation group responsible for linking to the side chain of specific amino acid residues of the antibody, (2) a drug release trigger that determines how the payload is released in response to different conditions, and (3) spacers between the trigger and the bioconjugation group, which serve as junctions and can impact linker parameters like hydrophilicity.
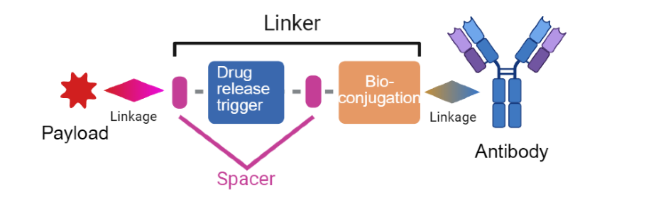
Figure 1. Schematic diagram of a typical ADC linker structure [1]
Linkers are classified based on their payload release mechanism:
• Cleavable Linkers: These contain labile bonds sensitive to tumor microenvironment (TME) conditions or intracellular factors (e.g., proteases, low pH, high glutathione levels). When triggered, they release the active payload at the tumor site.
• Non-Cleavable Linkers: These are stable in circulation, releasing the payload only when the antibody is degraded within the lysosome.
Due to their tumor-selective activation profile, cleavable linkers are currently the dominant approach in ADC development.
Classic Designs and Advances in Cleavable Linkers
1. Enzyme-Labile Linkers
These linkers exploit the high expression of specific enzymes, such as lysosomal cathepsin B, to enable targeted and controlled payload release.
• Classic Design: The valine-citrulline (Val-Cit) dipeptide, often paired with the self-immolative spacer p-aminobenzylcarbamate (PABC), is the archetype. Cathepsin B cleaves the dipeptide, triggering PABC breakdown and releasing the unmodified payload, e.g., monomethyl auristatin E (MMAE). This design underpins ADCs like brentuximab vedotin.
• Innovative strategies: Recognizing limitations in plasma stability observed with Val-Cit in preclinical models (potentially confounding PK/PD translation), researchers have developed more stable tripeptide linkers. Examples include glutamic acid-valine-citrulline (EVCit) and glutamic acid-glycine-citrulline (EGCit). These modifications significantly enhance systemic stability, widening the therapeutic index.
2. Acid-Labile Linkers
These linkers exploit differences in pH across normal tissues, the tumor microenvironment, intracellular endosomes, and lysosomes to enable the selective release of drugs.
• Classic Design: Acid-labile functionalities include hydrazones (e.g., in gemtuzumab ozogamicin’s AcBut linker) and carbonate bonds (e.g., CL2A linker in sacituzumab govitecan, releasing SN-38 payload via hydrolysis).
• Innovative strategies: Novel acid-labile motifs have been investigated. A silyl ether-based moiety and crosslinker derived from the natural product gallic acid offer faster hydrolysis rates under acidic conditions and tunable stability profiles, providing greater flexibility for next-generation ADCs.
3. Redox-Labile Linkers
These linkers are designed to exploit the higher concentrations of glutathione (GSH) and reactive oxygen species (ROS) in tumor cells compared to normal cells, facilitating drug release under reductive or oxidative intracellular conditions.
• Classic Design: Disulfide bonds are the primary reductively cleavable group. Stable in circulation, they are rapidly cleaved intracellularly by high GSH concentrations. The sulfo-SPDB (N-succinimidyl 4-(2-pyridyldithio) butyrate) linker in mirvetuximab soravtansine exemplifies this approach.
• Innovative strategies: Research on disulfide bonds for linker design mainly focuses on steric hindrance to increase plasma stability. Furthermore, the diselenide bond represents a promising “dual-responsive” system, susceptible to cleavage by both GSH and ROS, potentially offering enhanced tumor selectivity.
Bioconjugation and Spacer Optimization
In addition to drug release triggers, optimizing the bioconjugation groups and spacers remains a critical strategy for improving ADC performance. Conventional methods based on random bioconjugation with cysteine and lysine have been thoroughly verified by the approved ADCs. However, the manufacturing of ADCs with conventional bioconjugation methods is challenging for achieving a consistent drug-to-antibody ratio (DAR) across batches. To address this issue, site-specific conjugation has been developed. This technology enables precise attachment of the linker to specific sites on the antibody, leading to more homogeneous ADCs. It guarantees high consistency of the DAR across batches.
The spacer, bridging the bioconjugation group, drug release trigger, and payload, plays a significant role in influencing the hydrophilicity, drug release, and branching of the linkers. Optimizing the spacer is crucial for improving the pharmacokinetics and pharmacodynamics of the ADC.
• Spacer for Enhanced Hydrophilicity: Introducing hydrophilic groups into the spacer can help reduce the propensity for ADC aggregation and improve formulation stability.
• Spacer for Branched Linker: The branched linker enables a combinatorial strategy of different payloads. For example, branched linkers can carry both a cytotoxic drug and an immune modulator, enabling synergistic mechanisms of action and potentially circumventing resistance.
One-Stop ADC Linker and Payload Synthesis Capability Platform
The increasing complexity of modern linker design—spanning novel triggers, multifunctional spacers, and intricate branched structures—demands sophisticated chemical synthesis capabilities. Translating innovative concepts into high-quality, developable drug candidates requires robust and flexible platforms.
WuXi AppTec Research Chemistry Services (RCS) exemplifies this commitment, offering a comprehensive one-stop ADC linker-payload solution of:
• Reliability: With over a decade of ADC expertise, RCS has delivered more than 27,000 compounds.
• Diversity: Access to a vast library of over 1,000 linkers covering over 30 attachment sites and novel payloads, enabling rapid modular assembly.
• Flexibility: RCS’s platform supports scalable synthesis from milligrams to hundreds of grams, offering various flexible business models, including Full-time Equivalent (FTE) or Fee-for-Service (FFS).
• Strong Support: Equipped with efficient separation and purification, comprehensive analytical support (LC-MS, NMR), and high-potency laboratories to ensure the safe handling and high-quality delivery of high-potency, challenging molecules.
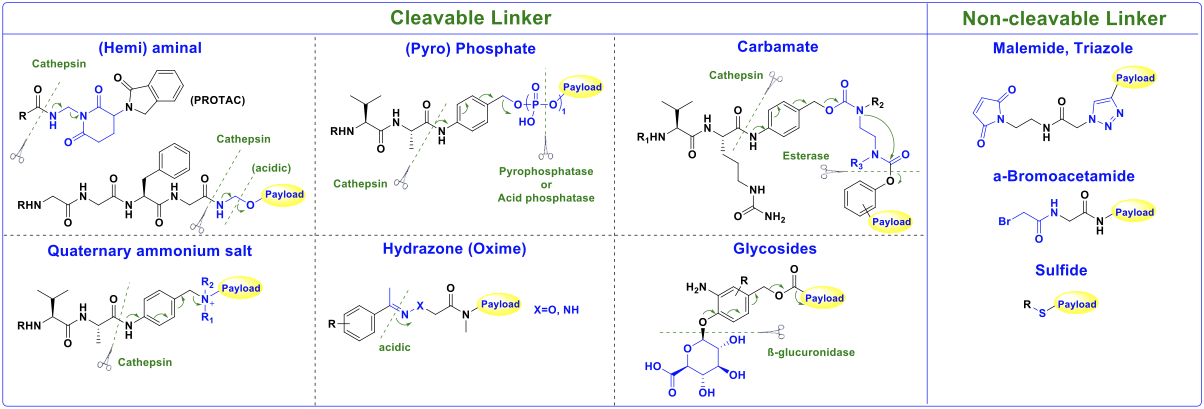
Figure 2. WuXi AppTec RCS’s accumulated experience across a wide range of linker synthesis
Driving the Next Wave of ADC Therapeutics
As the field advances toward next-generation ADCs, linker design will remain a critical enabler of both efficacy and safety. WuXi AppTec RCS is dedicated to being your trusted partner in ADC discovery, leveraging its professional, efficient, and integrated capabilities in chemical synthesis, analysis, and purification to accelerate next-generation ADCs from concept to clinic, ultimately bringing transformative therapies to patients worldwide.
Disclaimer: This article is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
Reference:
1. Linker Design for The Antibody Drug Conjugates: A Comprehensive Review, ChemMedChem 2025, e202500262
Disclaimer: This presentation is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds/technologies mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
CAPABILITIES
How can we help?
Get in touch with an expert.