WuXi Biologics
Offering End-to-End Solutions
From “Escaping from Flatland” to Kilogram-Scale Production: The Evolution of Photochemistry in Drug Discovery and Development
From “Escaping from Flatland” to Kilogram-Scale Production: The Evolution of Photochemistry in Drug Discovery and Development
With the widespread adoption of LED technology, the maturation of photocatalysis mechanisms, and the advancement of flow chemistry, photochemistry has evolved from a niche method—once perceived as complex and narrowly scoped—into an indispensable and transformative force in modern drug discovery.
Photochemistry enables the efficient synthesis of intricate organic structures and significantly expands the chemical space accessible to medicinal chemists. This article, referencing the Journal of Medicinal Chemistry Perspective ‘Photochemistry in Medicinal Chemistry and Chemical Biology’, systematically outlines the applications and cutting-edge progress of photochemistry across the drug R&D stage, as well as strategies for breaking through the bottlenecks in large-scale production.
Photochemistry: Transcending the Limitations of Traditional Medicinal Chemistry
1. Reconstructing Synthetic Logic: “Escaping from Flatland” under Mild Conditions
Recent analyses of approved drug molecules reveal a positive correlation between the fraction of sp3-hybridized carbon atoms (Fsp3) and key physicochemical parameters as well as success rates in clinical trials (Figure 1). Conversely, molecules rich in aromatic rings or sp2-hybridized fragments often encounter physicochemical liabilities during the transition from preclinical research to clinical development, such as low aqueous solubility, rapid metabolic clearance, or challenging off-target profiles.
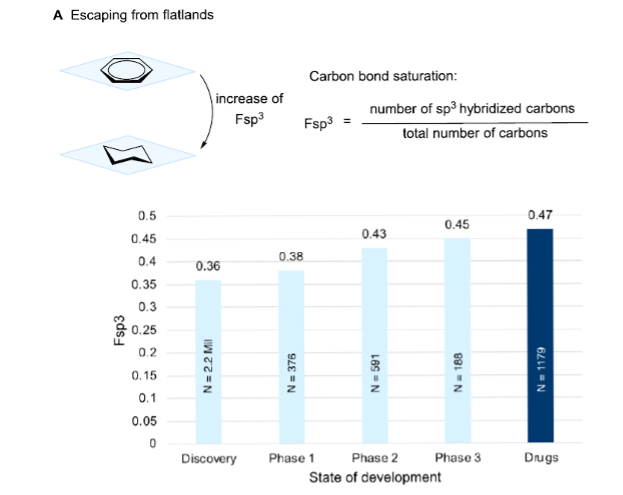
Figure 1. Fraction of sp3-hybridized carbon atoms that correlates with success in clinical studies. [1]
Traditional thermochemical reactions are often governed by thermodynamic control, making it difficult to effectively explore unique 3D chemical spaces. The introduction of photochemistry provides a novel synthetic logic and viable pathway for constructing aliphatic frameworks with higher sp3 content and three-dimensional complexity—achieving the so-called “Escape from Flatland”.
2. Methodological Strategies and Technical Applications in Photochemistry
Photochemistry’s most compelling feature is its ability to harness light energy to promote substrates from ground states to excited states, thereby driving thermodynamically disfavored or kinetically hindered transformations. Modern photochemistry focuses on the efficient and sustainable preparation of high-value molecules while minimizing ecological and economic footprints. Generally, photochemical strategies are categorized into two types:
- Direct Photochemical Excitation of Reactants: This approach requires the reactants to absorb the light used for chemical transformation, while the products remain stable under irradiation (Figure 2A).
- Use of Photocatalysts (PC) or Photosensitizers (PS): These species absorb light and interact with the substrate via Single Electron Transfer (SET) or Energy Transfer (EnT), accompanied by the quenching of the excited state of the PC (Figure 2B). This strategy allows the use of selective visible light to initiate a diverse range of downstream transformations.
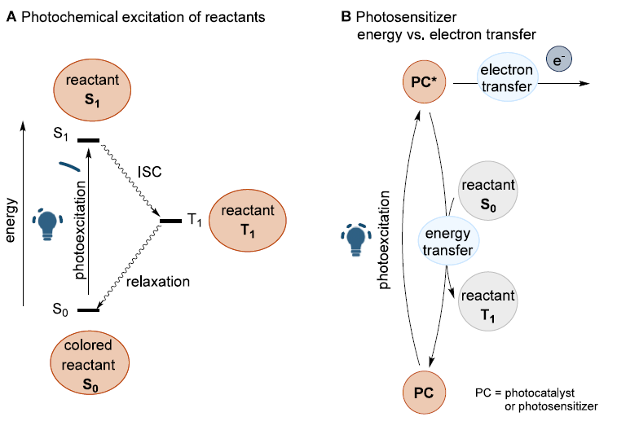
Figure 2. Different photochemical strategies. (A) Direct photochemical excitation of reactants. (B) Photosensitizer-mediated excitation. [1]
- Applications of Direct Excitation Strategies
Classic examples of direct photoexcitation include [2+2]-cycloadditions of olefins and the Paternò–Büchi reaction involving carbonyls (Figure 3A). However, these typically require high-energy UV light, which limits their scope as many substrates are UV-sensitive or prone to undesired decomposition.
In contrast, colored reactants can be directly excited by visible light, offering broader utility in fields such as photoswitches, materials, or photosensitive protecting groups. From a synthetic perspective, the visible light-mediated photosensitization of colored diazo compounds (4) or nitrene precursors (8) has gained significant attention to access reactive carbene (5) or nitrene (9) intermediates to forge cyclopropanes, new X-H bonds, aziridines, or C-N bonds with high efficiency (Figure 3B).
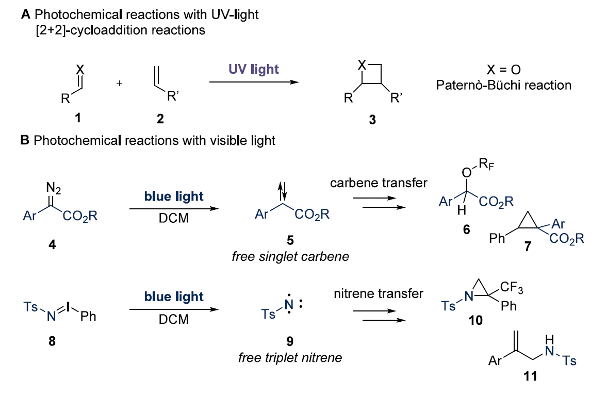
Figure 3. Examples of direct excitation of reagents [1].
- Applications of Photosensitizers for Electron/Energy/Hydrogen Transfer
Light irradiation of a PS or PC to its excited state and its application in synthesis has received significant interest over the past 15 years. Advanced systems integrate these with transition-metal catalysts (e.g., Ir, Ru, Ni) to access oxidation states that are difficult to reach under conventional conditions. Recent research has also investigated the fusion of photocatalysis with traditional C–H functionalization, where catalysts exhibit distinct reactivity upon photoexcitation relative to their ground state. To date, these strategies have successfully facilitated the construction of vital C(sp²)–C(sp³) and C(sp³)–C(sp³) bonds (Figure 4).
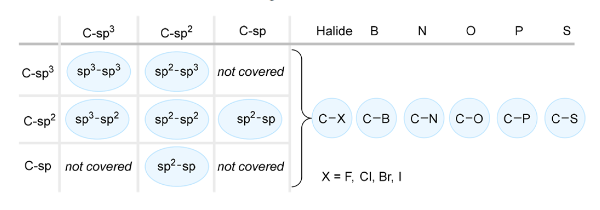
Figure 4 Photochemical Carbon−Carbon or Carbon−Heteroatom Bond Forming Reactions [1]
- Metallaphotoredox Catalysis Strategies
While traditional photochemistry provides tools for retrosynthetic disconnections, its full potential in radical chemistry was realized only with the advent of photoredox catalysis. By modulating the electronic properties of transition metal catalysts, chemists can selectively generate and capture radical intermediates. This has become an indispensable tool for medicinal chemists, enabling new bond-forming patterns such as the deoxygenative cross-coupling of alcohols, decarboxylative coupling reactions, and site-selective C–H functionalization.
- Applications of Photochemistry in Drug R&D
1. Target Identification and Validation
Chemical biology plays a pivotal role in drug discovery by utilizing chemical tools to analyze and affect biological systems. Within this field, photochemical biology has emerged as a highly promising direction due to its non-invasive activation and spatiotemporal control. Photosensitive chemical probes can be specifically activated by light to achieve precise modulation of complex biological systems within predefined spatial and temporal windows. Typical applications include photoswitches, photoclick reactions, and photocages.
Furthermore, these tools facilitate the identification of unknown biological targets for ligands or the selective labeling of biomacromolecules in proximity to a protein of interest within specific organelles—techniques known as photoaffinity labeling (PAL) and photocatalytic proximity labeling (Figure 5).
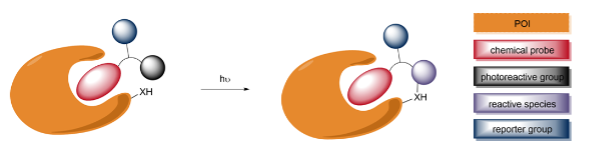
Figure 5. Labeling of biomolecules by photoaffinity probes. [1]
2. Hit Discovery
Photochemistry demonstrates unique potential in hit discovery. Light-activated reactions enable the rapid construction of structurally diverse small-molecule libraries, significantly expanding the chemical space available for activity screening and supporting early-stage structure-activity relationship (SAR) studies. Specifically, multicomponent photochemical reactions and photocatalysis allow for the efficient synthesis of scaffold-rich libraries for high-throughput screening. Moreover, photochemical methods have significantly advanced the synthesis of DNA-encoded libraries (DELs) by enhancing scaffold diversity, thereby increasing the probability of identifying high-quality hits.
3. Lead Optimization
During the hit-to-lead optimization process, the core objective is to identify and optimize small molecules that can modulate a specific biological target. This process typically involves the synthesis and evaluation of numerous analogs to identify compounds with improved potency, selectivity, and pharmacokinetic (PK) profiles.
Photochemistry has become a vital tool in this stage, enabling the efficient construction of complex architectures with unique chemical and biological properties. Its primary advantage lies in the facile synthesis of diverse structures that are challenging to access via traditional synthetic methods. Examples include the construction of novel scaffolds for bioisosteric replacement, the expansion of sp3-rich frameworks through stereochemical control and photoisomerization, and late-stage functionalization (LSF) to introduce key groups without disrupting the core molecular framework.
- Flow Photochemistry: Addressing the Challenges of Process Scale-up
While photochemistry has advanced rapidly in academia, its adoption in the pharmaceutical industry remains hindered by significant scale-up challenges. According to the Beer-Lambert Law, light intensity in a reaction medium decay exponentially with the path length. In industrial-scale batch reactors, this often leads to inhomogeneous irradiation, reduced reaction efficiency, and increased by-product formation.
The key to overcoming these limitations is the adoption of continuous-flow technology. Flow photochemistry utilizes narrow-diameter tubing or microchannels to ensure uniform and efficient irradiation, thereby avoiding the light attenuation issues inherent in batch scale-up. In recent years, flow photochemistry has achieved numerous breakthroughs; for instance, many photochemical rearrangements for the generation of privileged scaffolds can produce intermediates or active pharmaceutical ingredients (APIs) with higher yields and shorter residence times.
- RCS Photochemistry Platform: Empowering Drug R&D
WuXi AppTec’s Research Chemistry Services (RCS) has systematically built an integrated photochemistry platform. By consolidating high-throughput screening (HTS), process optimization, and flow chemistry, the platform addresses bottlenecks across the entire workflow—from milligram-scale exploration to kilogram-scale production.
1. Technical Capabilities
- Reaction Types: Over 100 reaction types (Figure 6), including C(sp2)–C(sp3) and C(sp3)–C(sp3) coupling, decarboxylative functionalization, and cycloaddition.
- Instrumentation: Over 100 commercial and integrated reactors, covering multiple wavelengths.
- Catalyst Library: A stock of over 60 photocatalysts (Ru, Ir, and organic dyes).
- High-Throughput Experimentation (HTE): 96-well photoreactors for rapid identification of optimal reaction conditions.
- Scale-up: An established “HTE + Flow” model enables direct scale-up from mg scale to Kg scale (1–10 kg/day).
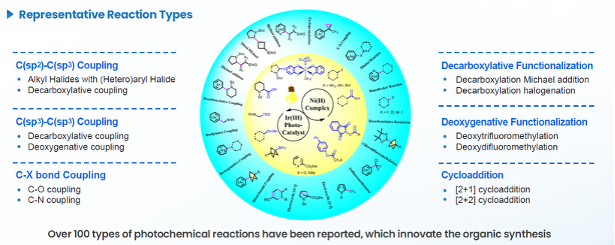
Figure 6. Diversified photochemical reaction types delivered by RCS
2. Case Study
A client project required the construction of a multi-substituted chiral cyclohexane as a key scaffold. Conventional processes exhibited low yields and poor cis/trans selectivity (~1:1). The RCS team introduced photochemical processes and adopted a combination of “HTE+Flow” to shorten the conventional 5-step thermochemical route to a flow photochemical route that only requires 1 step. A 100g+ scale synthesis was completed in just 9 hours with good yield and high cis-selectivity, significantly improving process efficiency and scalability (Figure 7).
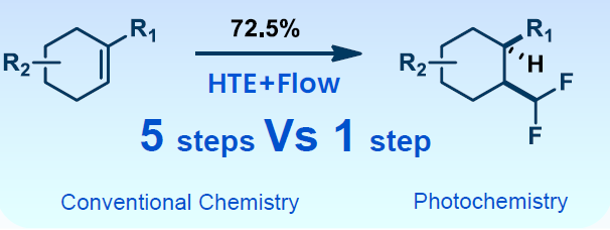
Figure 7. Flow photochemistry achieving 100g-scale scale-up
- Outlooks
Photochemistry is profoundly transforming the landscape of medicinal chemistry. As the technology matures, it will become an indispensable tool for medicinal chemists. RCS is committed to translating this “light magic” into reality, accelerating the drug R&D process for global partners.
Reference:
[1] Photochemistry in Medicinal Chemistry and Chemical Biology J. Med. Chem. 2024, 67, 4322−4345
Disclaimer: This article is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
CAPABILITIES
How can we help?
Get in touch with an expert.