WuXi Biologics
Offering End-to-End Solutions
Magical Power of Quantum Mechanics
LUMO Analysis of Electrophiles (Part II)
QM Magic Class | Chapter 7
Our introductory chapter 1 on LUMO leaves us with an intriguing question, that is, when the 5-CH3 group in dichloropyrimidine A is replaced with Cl, Br, CF3, and CCl3, which chloro group will be displaced by an aliphatic amine? In this chapter, we will discuss the orbital analyses of these pyrimidines.

Table 1. Nucleophilic substitution of substrate A with methylamine
LUMO and LUMO+1 in Nucleophilic Substitutions
First, we would like to introduce LUMO+1, which is the second Lowest Unoccupied Molecular Orbital, a level higher in energy than LUMO. Its involvement in reaction could become significant when energy gap between LUMO and LUMO+1 is small.
Shown on Figure 1 are LUMO/LUMO+1 orbitals and their corresponding energy levels of five dichloropyrimidine substrates. In the 5-CH3 substituent, LUMO lobes are located on C-4 and C-6 positions, accounting for their interactions with incoming nucleophilic reagents discussed in Chapter 1. Addition of methylamine at C-6 is reversible, whereas addition at C-4 is irreversible, leading to selective displacement of the chloro group observed. Energy gap between LUMO and LUMO+1 for this substrate is big enough that LUMO lobe distribution is enough to account for the observation.
Similar orbital and energy patterns can be observed for the 5-Br and 5-Cl substituents, accounting for the C-4 selective displacement observed. No surprise.
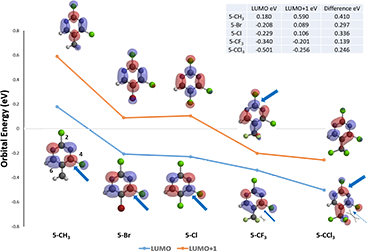
Figure 1. LUMO and LUMO+1 orbitals and corresponding energy levels of substrate A with various 5-substitutents
When it comes to the 5-CF3 substituent, the distribution of LUMO lobes has changed. Now the C-2 position is partially covered with LUMO lobe, enabling the substrate to potentially interact with incoming nucleophiles at this position. In addition, the energy levels between LUMO and LUMO+1 has become closer together, and the 5-CF3 group imposing higher degree of steric hindrance to incoming nucleophile, accounting for a 1:1 mixture of C-2 and C-4 substitution products observed.
With the 5-CCl3 dichloro-pyrimidine, the energy gap between LUMO and LUMO+1 is back to the normal range, we could focus our analysis on LUMO. This substrate has LUMO lobes on both C-2 and C-4, yet 5-CCl3 is substantially larger in size than 5-CF3, limiting accessibility to reaction at C-4, and led to selective displacement at C-2.
Plausible Reaction Mechanism Based on LUMO Analysis
5,6-Pyrimidyne was postulated as intermediate in the reaction of 4-methyl-5-bromopyrimidine B with sodamide (Figure 2), followed by deprotonation of the methyl group, and then amide addition to the resultant benzyne anion exclusively at C-6.[1]
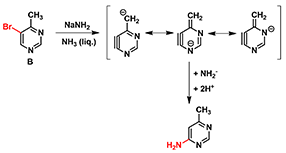
Figure 2. Amination of compound B: formation of a benzyne-like intermediate
We reasoned that it is not likely to have two anions reacting with each other and performed a QM analysis of the bromopyrimidine B.
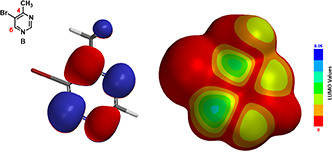
Figure 3. LUMO and LUMO Map of compound B
Shown on Figure 3 are the calculated LUMO and LUMO Map for bromopyrimidine B. There is little LUMO lobe associated with C-5, explaining why a SNAr substitution of the bromide with an amino group at C-5 was not observed. Both C-4 and C-6 have sizable lobes, yet LUMO Map indicates that C-6 is more accessible to nucleophilic attack (light blue). We reasoned that an amide anion is first added at C-6, leading to an intermediate anion shown in Figure 4, followed by an irreversible elimination of hydrogen bromide to generate 4-amino-6-methylpyrimidine.
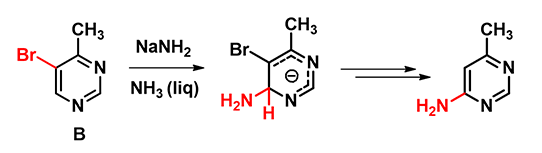
Figure 4. Amination of compound B: formation of an amino anion intermediate
To summarize, for nucleophilic substitution reactions on complex heterocycles, LUMO and LUMO map have been very useful for us to rationalize and predict reaction outcomes. When the energy gap between LUMO and LUMO+1 is small, it is important to consider both orbitals.
Inclusion of steric consideration in such analysis provides further insight to the reaction of interest.
Building on What We Just Learned
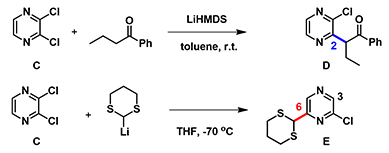
Reaction on compound C with lithium enolate of phenylbutanone is known to provide C-2 substituted product D. However, in 2006 McDonald et al.[2] reported that treatment of C with lithio dithiane afforded a tele-substitution product E, that is, addition of dithiane moiety at C-6 followed by displacement of the chloro group across the ring at C-3. Shown below in Figure 5 are the LUMO/LUMO+1 and their corresponding maps calculated for compound C. We hope you will have as much fun as we did in solving this decade old chemistry puzzle.
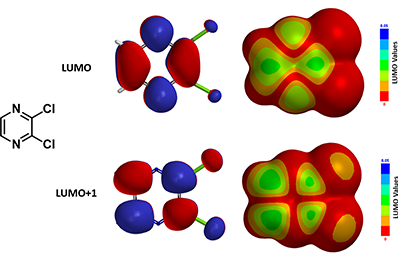
Figure 5. LUMO, LUMO + 1 lobe and corresponding LUMO map, LUMO + 1 map of compound C
References:
[1] J. A. Joule & G. F. Smith (1972). Heterocyclic Chemistry 2nd Edition. Page 131.
[2] J. E. Torr, J. M. Large, P. N. Horton, M. B. Hursthouse and E. McDonald. Tetrahedron Lett. 2006, 47, 31-34.
