WuXi Biologics
Offering End-to-End Solutions
“Catch the Flow”: Unlocking the Potential of Flow Chemistry in Early-Phase Drug Discovery
“Catch the Flow”: Unlocking the Potential of Flow Chemistry in Early-Phase Drug Discovery
Flow chemistry has revolutionized chemical synthesis by enabling continuous, precisely controlled reactions that offer significant advantages over traditional batch methods. This approach provides fine control over reaction parameters such as temperature, pressure, and residence time, enhancing safety and efficiency in early-phase research [1].
In flow chemistry, reagents are continuously pumped through dedicated reactors, where they mix and react under precisely controlled conditions. The reaction mixture moves through the system and is collected at the outlet. When coupled to a suitable process analytical technique (PAT) device, real-time monitoring and optimization can be achieved, especially in a high-throughput screening fashion (Figure 1) [2].
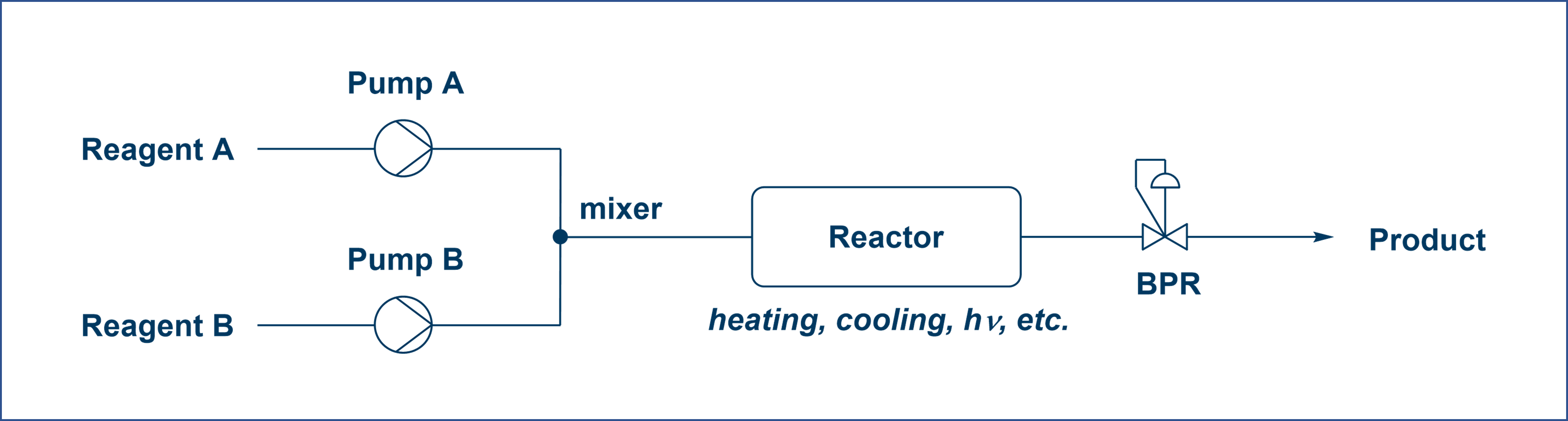
Figure 1. Schematic illustration of flow chemistry
Types of Flow Reactors
The operational principles of flow chemistry fundamentally depend on reactor design, with the seamless integration of pumps, back pressure regulators (BPRs), and various sensors. Selecting the appropriate reactor is crucial as it directly impacts a system’s ability to control reaction conditions, scale processes, and enable novel chemistry. Here’s a quick guide to the main types of reactors (Figure 2):
1. Microreactors (Chip Reactors) – Characterized by channels with dimensions in the micrometer range allowing rapid mixing. Their high surface-area-to-volume ratio enables rapid mixing and heat transfer, making them particularly suitable for exothermic reactions and hazardous transformations such as diazo or azide chemistry, which require precise control to prevent uncontrolled gas evolution or accumulation of unstable intermediates [3].
2. Tubular/Coil Reactors – Constructed from materials like PFA, PTFE, or stainless steel, these versatile and durable reactors are the most frequently used in daily work. They provide near-plug flow behavior, minimizing back-mixing and allowing better control over reaction kinetics, although some axial dispersion may occur. One particular application is in flow photochemistry, enabling uniform irradiation leading to smooth up-scaling with enhanced overall efficiency [4].
3. Packed-Bed Reactors – Used for heterogeneous catalysis (e.g., hydrogenation) and biotransformation with immobilized enzymes, aligning with sustainable chemistry trends. Packed-bed reactors simplify catalyst screening in flow, enabling rapid evaluation of immobilized catalysts for hydrogenation or biotransformation [5].
4. Continuous Stirred Tank Reactors (CSTRs) – While recognized as a hybrid approach of flow and batch, CSTRs are valuable for slurries and multiphase systems, maintaining uniform composition in continuous setups.
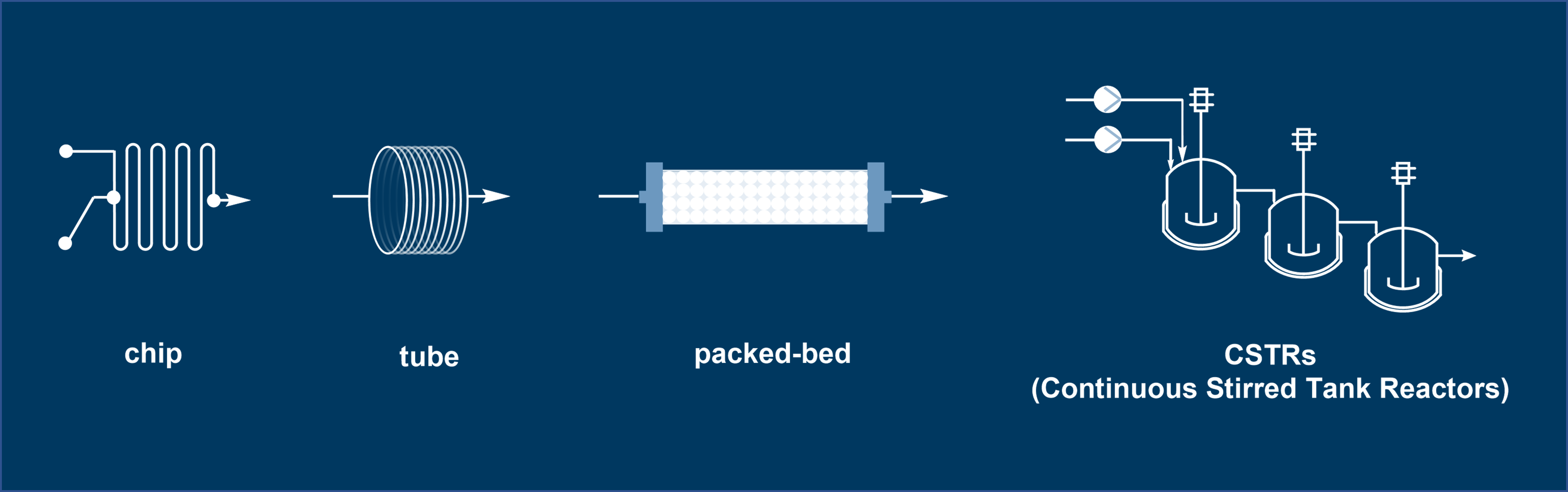
Figure 2. Types of flow reactors
Advantages and Challenges of Flow Chemistry
The transformative impact of flow chemistry stems from several key advantages:
• Enhanced Mass/Heat Transfer – The high surface-area-to-volume ratio in flow reactors enables efficient heat dissipation and mixing, improving safety and reaction control [7].
• Improved Safety – Small reactor volumes reduce risks of the accumulation of hazardous substances. For example, flow systems enable safe on-demand generation of diazonium intermediates, minimizing risks of uncontrolled decomposition [8].
• Access to Extreme Conditions – Flow systems safely enable high-pressure/temperature reactions. For example, a Friedel-Crafts acylation step in ibuprofen synthesis achieved >80% yield in minutes under flow conditions, leveraging precise thermal control to mitigate exothermic risks and reduce catalyst loading compared to batch methods [9].
• Scalability – Flow chemistry enables rapid optimization of reaction conditions at a small scale, with the potential for later translation to larger scales via numbering-up strategies while maintaining optimal reaction conditions [10].
Despite these advantages, flow chemistry presents certain challenges. Fouling and clogging in reactors [11], often caused by precipitation, can block channels and require optimized reaction conditions. Additionally, cost and training barriers exist due to the high initial investment in pumps, reactors, and sensors [12].
Applications of Flow Chemistry in Early-Phase
1. Photochemical Reactions: Better Light Distribution and Faster Reactions
In photochemical reactions, batch reactions struggle with uneven light exposure, leading to inefficient use of energy and lower yields. Flow reactors address this issue by using narrow tubes that allow light to pass through more effectively, ensuring uniform exposure of all reactants [4].
For example, a bromination reaction can be scaled up to 1.1 kg in 90 min using flow photo conditions with a yield of 75% (Figure 3A). Similarly, a photocatalytic reaction that was difficult to scale up in batch could deliver 50 g in 3 h (Figure 3B). This demonstrates how continuous flow ensures more efficient light use, accelerates reactions, and increases productivity.
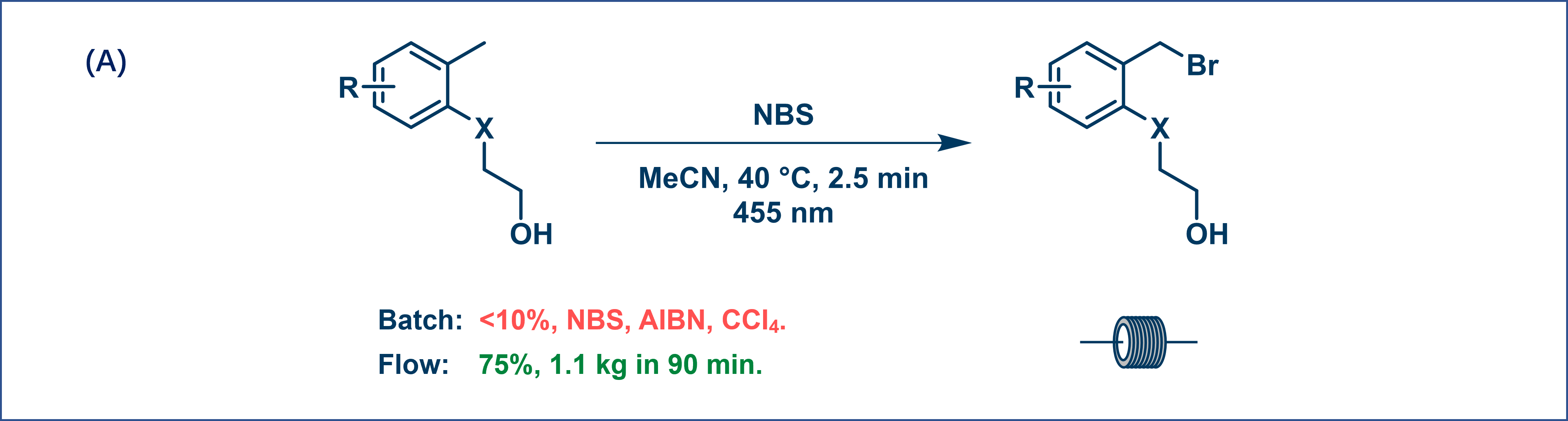
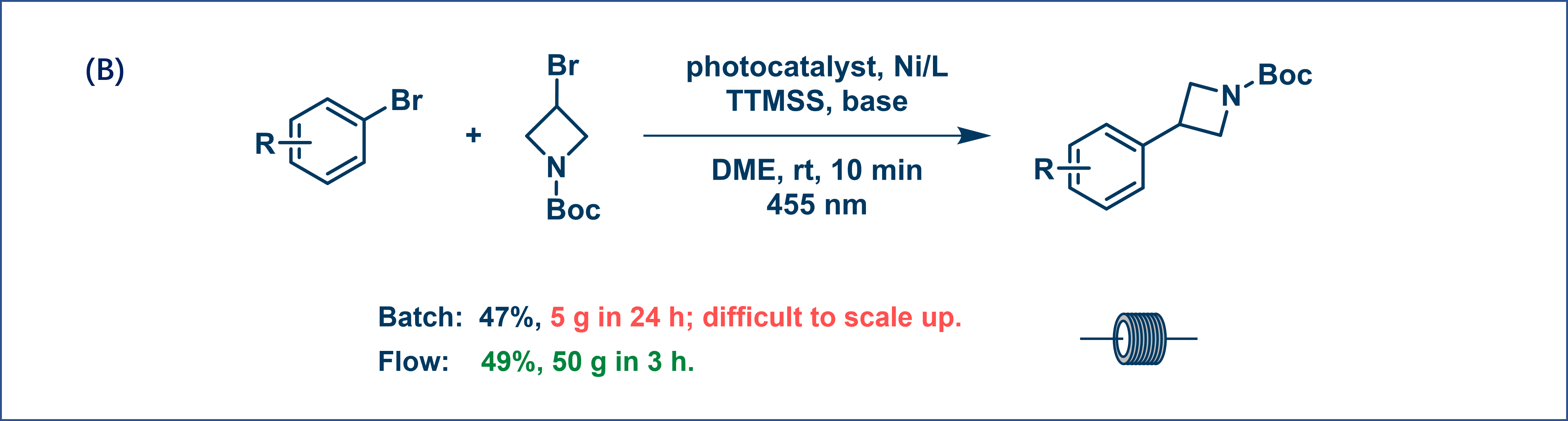 Figure 3. Photochemical reactions
Figure 3. Photochemical reactions
2. Hydrogenation: Safer, More Efficient Gas Reactions
Hydrogenation reactions, which reduce unsaturated compounds, often require handling high-pressure hydrogen gas in large batch reactors. Flow chemistry makes this process safer by confining reactions to small compact reactors where hydrogen is introduced in controlled amounts [5].
For instance, a hydrogenation reaction that yielded only 49% in batch with side reactions achieved a 95% yield without undesirable side products when conducted in flow (Figure 4).
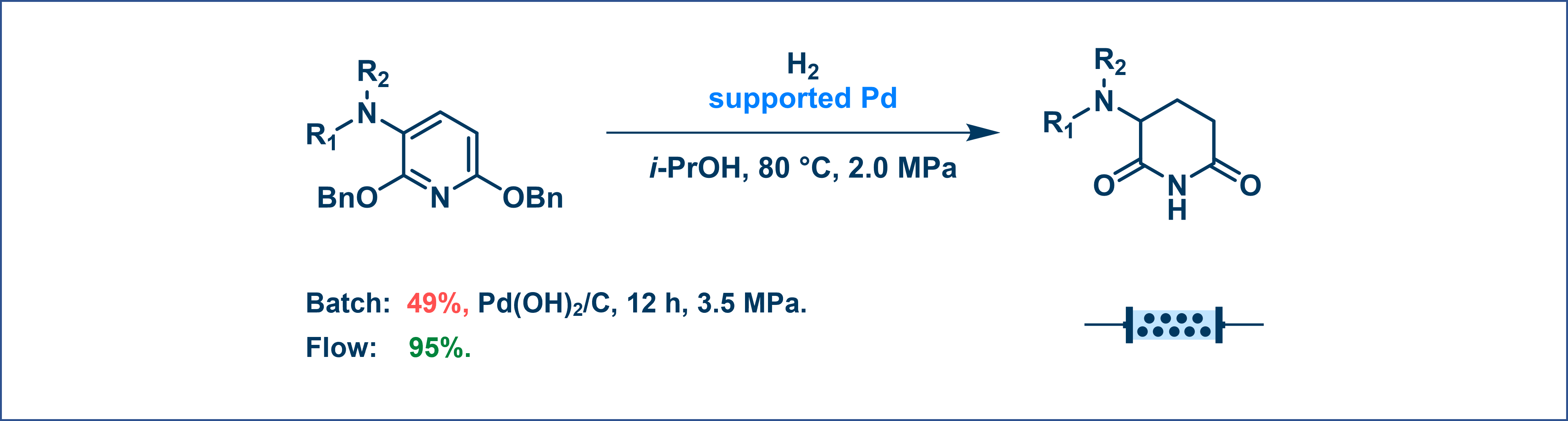 Figure 4. Hydrogenation reaction
Figure 4. Hydrogenation reaction
3. Organolithium Reactions: Speed and Safety with Highly Reactive Reagents
Organolithium reagents like n-BuLi are highly reactive and typically require low temperatures in batch reactions to prevent the decomposition of the lithiated species. Flow reactors handle these reagents more safely through rapid mixing and short residence times, with minimal accumulation of intermediates [13].
A reaction using n-BuLi that yielded only 32% in batch at −78°C was carried out in flow at −20°C, resulting in a 60% yield. This shows how flow chemistry lets chemists work with highly reactive intermediates at milder temperatures with improved throughput (Figure 5).
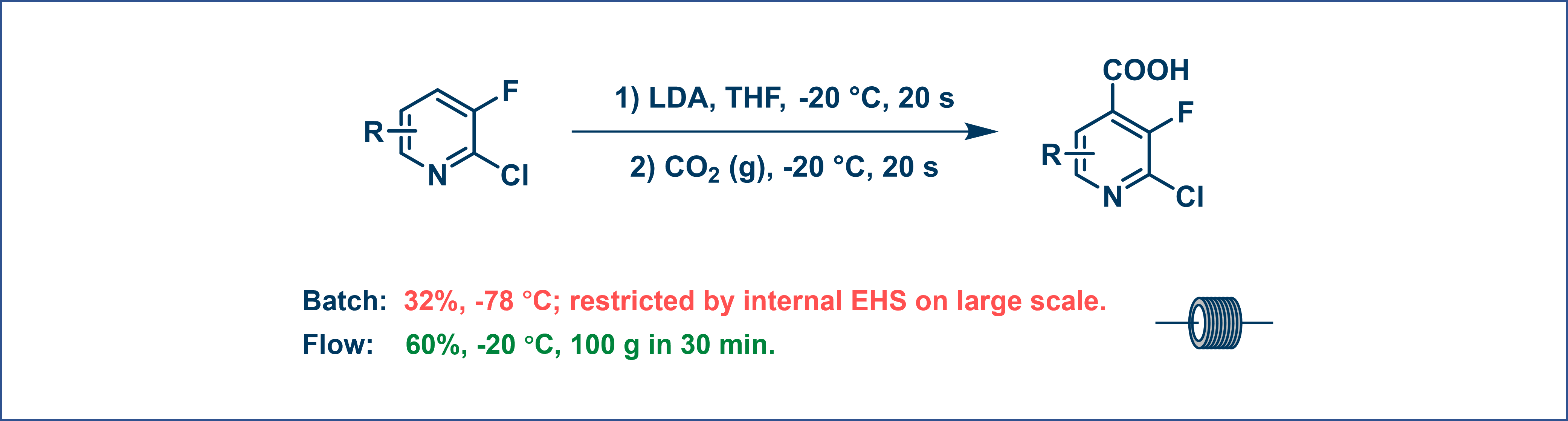 Figure 5. Organolithium reaction
Figure 5. Organolithium reaction
4. Diazotization: Safer and More Efficient Intermediate Handling
Diazotization reactions generate highly unstable diazonium salts that can be explosive in large quantities. Batch setups require a slow, small-scale generation to avoid explosions. In flow, however, the diazonium intermediate is generated on-demand and immediately used, so there’s a minimal accumulation of unstable substances [3].
A diazotization reaction that yielded only 56% in batch achieved a 90% yield and 1 kg of product in just 8 h when conducted in flow (Figure 6). This process not only improved reaction yield but also made the process safer by eliminating the risk of an uncontrolled diazonium intermediate buildup.
 Figure 6. Diazotization reaction
Figure 6. Diazotization reaction
5. Hydride Reductions: Improved Control Over Exothermic Reactions
Hydride reductions, such as those using LiAlH₄ or NaBH₄, are highly exothermic and require careful control to prevent runaway reactions. Flow systems prevent heat buildup through continuous mixing and efficient heat dissipation. This is particularly important when using strong reducing agents that could decompose or cause explosions if added too quickly [14].
A selective nitro reduction employing B₂(OH)₄/4,4′-bipy in DMF is highly reactive, exothermic, and gas-evolving. Compared to batch processing, flow provides superior thermal control and safer operation, while effectively suppressing side reactions such as aryl deiodination. Collectively, these advantages boost the isolated yield to 96% (Figure 7).
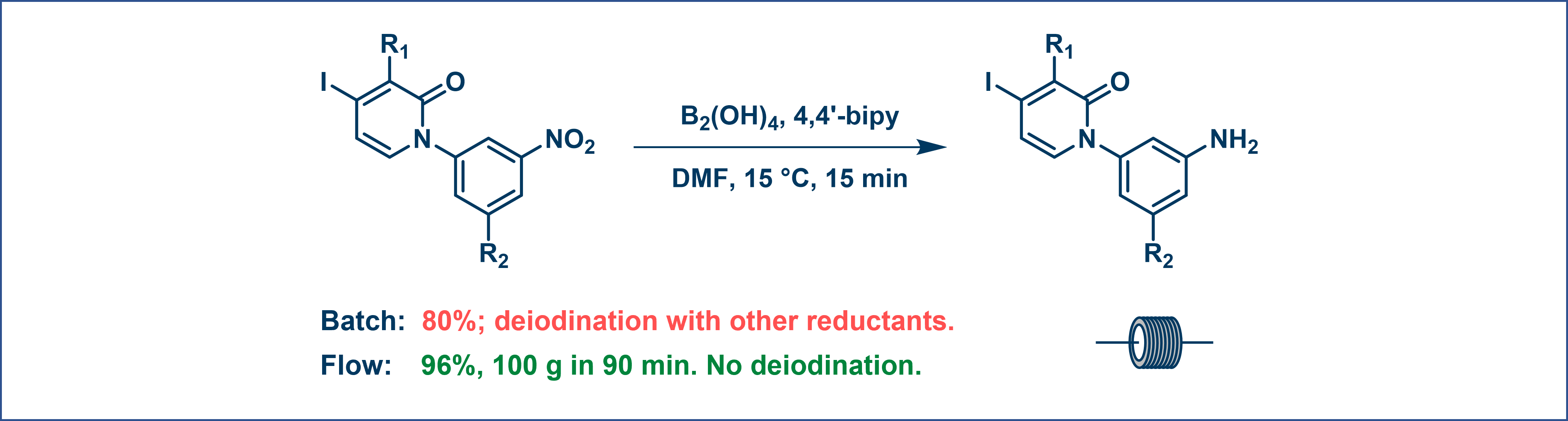 Figure 7. Hydride reduction
Figure 7. Hydride reduction
6. High-Temperature Reactions: Pushing Beyond Batch Limits
Flow reactors uniquely enable safe access to extreme reaction conditions, such as high temperatures and pressures, that are highly hazardous or unattainable in traditional batch processes [15]. For instance, a Boc-protected amine successfully undergoes thermal deprotection (de-Boc) specifically under high-temperature flow conditions (250 °C, 10 min, 4.0 MPa). Traditional batch chemistry relying on acid-mediated methods fails here due to the facile elimination of the sensitive tertiary alcohol functional group (Figure 8). This unique capability makes an otherwise challenging transformation feasible, demonstrating how flow chemistry can overcome limitations inherent to conventional batch processing.
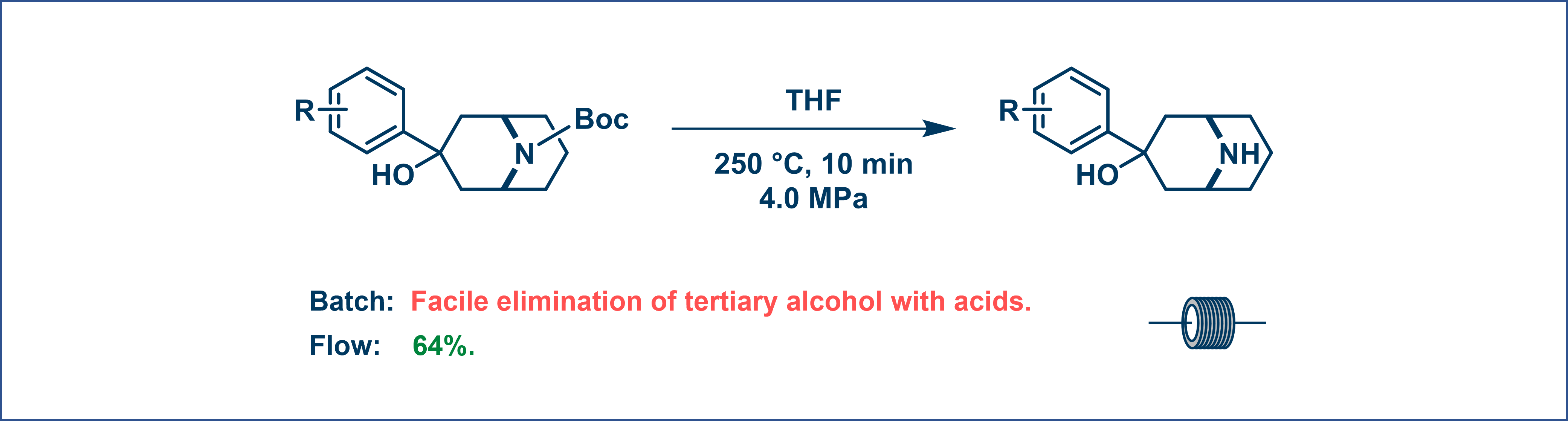 Figure 8. High-temperature reaction
Figure 8. High-temperature reaction
7. Oxidation Reactions: Precise Control for Scalable Synthesis
Oxidation reactions requiring precise control often face scalability and safety challenges. Flow chemistry improves temperature management, mixing efficiency, and reagent handling, enabling successful scale-up while maintaining reaction integrity [16].
A Swern oxidation reaction typically requires cooling down to -78°C in batch processes. However, with flow chemistry, the reactive intermediate is generated and consumed readily, allowing this step to proceed at just -5 °C for 1 min to generate the intermediate. Subsequently, the secondary alcohol was pumped in at -5 °C to complete the oxidation. This method enables us to oxidize 143 g in 1 h, significantly enhancing both energy and time efficiency (Figure 9).
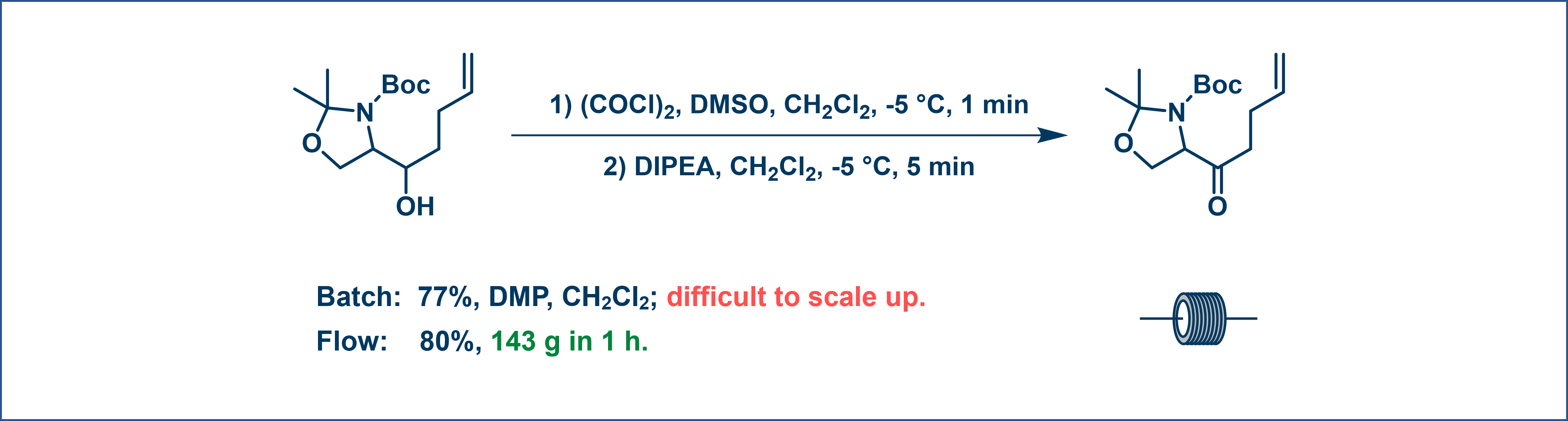 Figure 9. Oxidation reaction
Figure 9. Oxidation reaction
8. Hazardous Reagents: Safer and More Efficient Use
Flow chemistry safely handles hazardous reagents like nitric acid, azides, and diazomethane, by generating small amounts for immediate use, minimizing explosion and/or exposure risks.
Nitration reactions requiring highly reactive mixtures are safer in flow, where nitrating agents are added gradually in small reactor volumes. Similarly, azide reactions are made more safely by instantaneous production and consumption, preventing dangerous buildup. This ability to work with explosive or toxic intermediates in a controlled manner is an important benefit of continuous flow [17].
9. Telescope Reactions: More Efficient, Less Waste
Flow chemistry excels in telescoping multi-step reactions into a single continuous process, eliminating intermediate workups and purifications [12]. For example, a telescoped reaction involving borohydride reduction and oxidation achieved an 82% overall yield in flow, compared to 45% in batch methods. This integration reduces waste and enhances productivity, making it ideal for complex syntheses with unstable or hazardous intermediates (Figure 10).
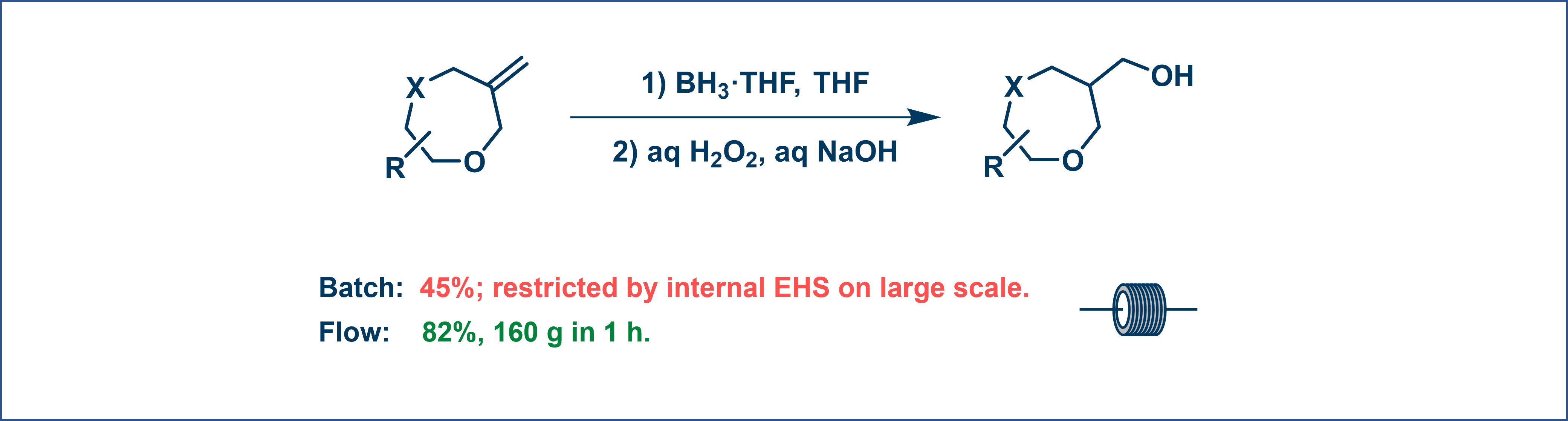 Figure 10. Telescope reaction
Figure 10. Telescope reaction
A Final Word
Flow chemistry is revolutionizing early-phase drug development by enabling safer, more efficient, and scalable synthetic processes, while also helping the expansion of chemical space by achieving impossible batch transformations. Since 2022, WuXi AppTec’s Research Chemistry Services (RCS) has built a comprehensive Flow Chemistry Platform, supported by dedicated teams across multiple locations. The platform has successfully conducted over 65,000 reactions, ranging from mg to kg scales. Our expertise covers a broad spectrum of reactions, offering customers end-to-end services from parameter optimization to reaction scale-up.
Contact: chemistry_service@wuxiapptec.com
References
1. Plutschack, M. B., Pieber, B., Gilmore, K., & Seeberger, P. H. (2017). The hitchhiker’s guide to flow chemistry. Chemical Reviews, 117(18), 11796 11893. https://doi.org/10.1021/acs.chemrev.7b00183
2. Fitzpatrick, D. E., Battilocchio, C., & Ley, S. V. (2016). Enabling technologies for the future of chemical synthesis. ACS Central Science, 2(3), 131–138. https://doi.org/10.1021/acscentsci.6b00015
3. Noël, T., Su, Y., & Hessel, V. (2016). Beyond organometallic flow chemistry: The principles behind the use of continuous-flow reactors for synthesis. Topics in Organometallic Chemistry, 57, 1–41. https://doi.org/10.1007/3418_2015_152
4. Cambié, D., Bottecchia, C., Straathof, N. J., Hessel, V., & Noël, T. (2016). Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chemical Reviews, 116(17), 10276–10341. https://doi.org/10.1021/acs.chemrev.5b00707
5. Hübner, S., Kressirer, S., Kralisch, D., Bludszuweit-Philipp, C., Lukow, K., Jähnisch, K., … & Schouten, J. C. (2012). Ultrasound and microstructures—A promising combination? ChemSusChem, 5(2), 279–288. https://doi.org/10.1002/cssc.201100540
6. McQuade, D. T., & Seeberger, P. H. (2013). Applying flow chemistry: Methods, materials, and multistep synthesis. The Journal of Organic Chemistry, 78(13), 6384–6389. https://doi.org/10.1021/jo400583m
7. Jensen, K. F. (2017). Flow chemistry—Microreaction technology comes of age. AIChE Journal, 63(3), 858–869. https://doi.org/10.1002/aic.15642
8. Movsisyan, M., Delbeke, E. I., Berton, J. K., Battilocchio, C., Ley, S. V., & Stevens, C. V. (2016). Taming hazardous chemistry by continuous flow technology. Chemical Society Reviews, 45(18), 4892–4928. https://doi.org/10.1039/C5CS00902B
9. Hartman, R. L., McMullen, J. P., & Jensen, K. F. (2011). Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis. Angewandte Chemie International Edition, 50(33), 7502-7519. https://doi.org/10.1002/anie.201004637
10. Elvira, K. S., Casadevall i Solvas, X., Wootton, R. C., & deMello, A. J. (2013). The past, present, and potential for microfluidic reactor technology in chemical synthesis. Nature Chemistry, 5(11), 905–915. https://doi.org/10.1038/nchem.1753
11. Gutmann, B., Cantillo, D., & Kappe, C. O. (2015). Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angewandte Chemie International Edition, 54(23), 6688–6728. https://doi.org/10.1002/anie.201409318
12. Lévesque, F., & Seeberger, P. H. (2012). Continuous-flow synthesis of the anti-malaria drug artemisinin. Angewandte Chemie International Edition, 51(7), 1706–1709. https://doi.org/10.1002/anie.201107446
13. Nagaki, A., & Yoshida, J. (2015). Extremely fast gas-liquid reactions in flow. Chemical Engineering & Technology, 38(2), 259–264. https://doi.org/10.1002/ceat.201400525
14. Wegner, J., Ceylan, S., & Kirschning, A. (2012). Flow chemistry: A key enabling technology for (multistep) organic synthesis. Advanced Synthesis & Catalysis, 354(1), 17–57. https://doi.org/10.1002/adsc.201100584
15. Baxendale, I. R. (2013). The integration of flow reactors into synthetic organic chemistry. Journal of Chemical Technology & Biotechnology, 88(4), 519–552. https://doi.org/10.1002/jctb.4012
16. Oelgemöller, M. (2016). Solar photochemical synthesis: From the beginnings of organic photochemistry to the solar manufacturing of commodity chemicals. Chemical Reviews, 116(17), 9664–9682. https://doi.org/10.1021/acs.chemrev.5b00720
17. Porta, R., Benaglia, M., & Puglisi, A. (2016). Flow chemistry: Recent developments in the synthesis of pharmaceutical products. Organic Process Research & Development, 20(1), 2–25. https://doi.org/10.1021/acs.oprd.5b00325
Disclaimer: This presentation is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds/technologies mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
CAPABILITIES
How can we help?
Get in touch with an expert.