WuXi Biologics
Offering End-to-End Solutions
Chemicals Insights | Struggling in C(sp2)-C(sp3) Bond Formation?Check This Out!
Chemicals Insights | Struggling in C(sp2)-C(sp3) Bond Formation?Check This Out!
Installing alkyl groups onto aryl rings will increase the degree of saturation in molecules and could modulate the biological, physicochemical, and pharmacokinetic properties of molecules to make them more drug-like. Given its vital role in synthesis, how can we form C(sp2)-C(sp3) bonds efficiently?
1. Conventional Methods
1.1 Two-Step Route
The two-step route involves a vinyl Suzuki coupling followed by hydrogenation (Figure 1a).
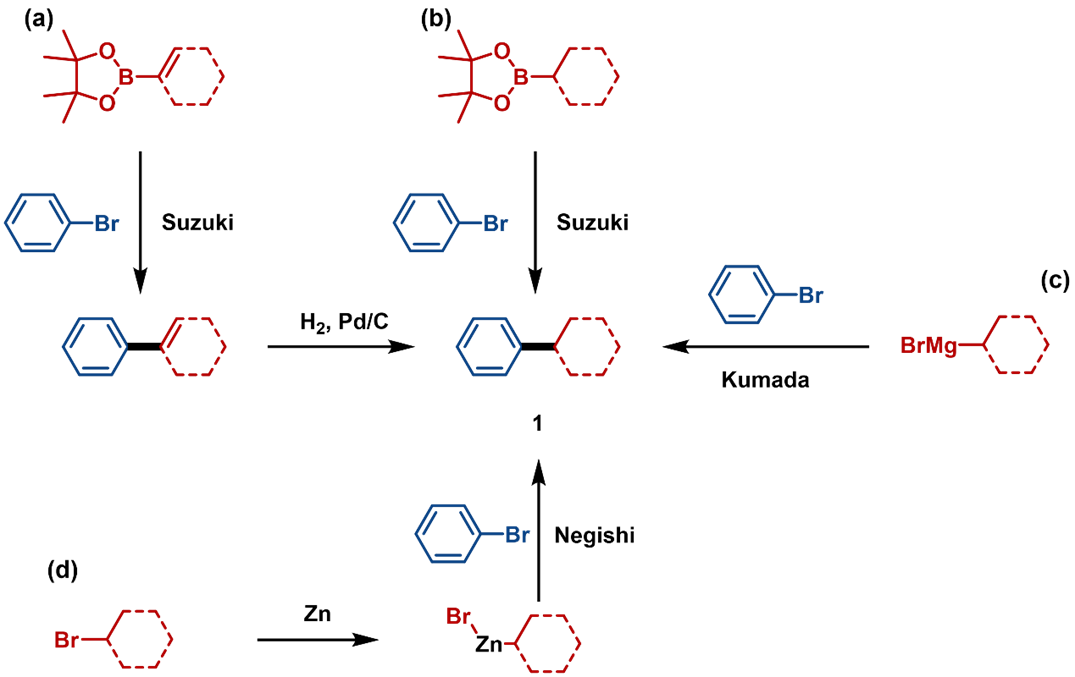
Figure 1. Conventional Methods for C(sp2)-C(sp3) Bond Formation
1.2 One-Step Route
One-step routes use direct C(sp2)-C(sp3) couplings, such as Suzuki-Miyaura (Figure 1b), Kumada (Figure 1c), Negishi coupling (Figure 1d), and Minisci reactions (Figure 2a).
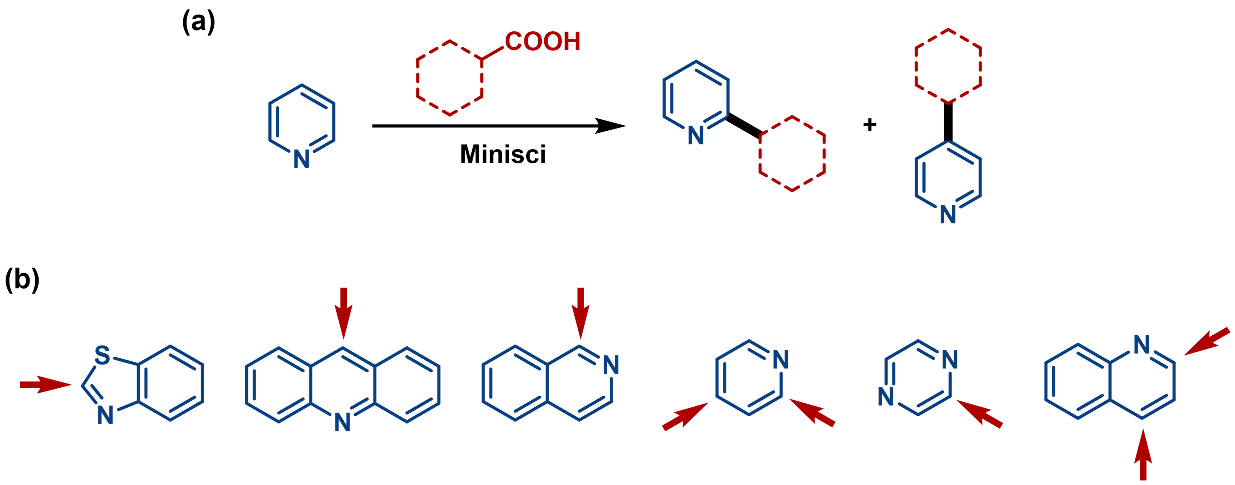
Figure 2. Minisci Reaction for C(sp2)-C(sp3) Bond Formation
Although these conventional methods are reliable and widely used in synthesis, they present some inevitable limitations.
1. Two-Step Route increases the design–synthesis–test (DST) cycle time.
2. One-Step Route: the carbon nucleophile reagents have limited commercial availability, and are frequently synthesized when needed. The inherent reactivity of the carbon nucleophiles and strong bases could limit functional group compatibility.
3. Minisci Reaction: the regiochemistry of the alkylation is dictated by the structure of the heteroarene (Figure 2b).
Some challenging groups, such as tertiary-alkyl, oxetane, azetidine and bridged rings are difficult to introduce using conventional methods.
Coming across these challenges in your practice? Ni-catalyzed coupling might be the solution. Let’s delve into it!
2. Ni-Catalyzed Reductive Cross Electrophile Coupling for C(Sp2)-C(Sp3) Bond Formation
The catalytic mechanism of conventional methods (such as Suzuki-Miyaura cross-coupling) has four steps: oxidative addition, metathesis, transmetalation, and reductive elimination (Figure 3). Although these methods are highly effective for C(sp2)-C(sp2) coupling, extension to Csp3 centers has proven to be challenging because of lower rates of transmetalation, and propensity of the alkylmetallic intermediates to undergo facile β-H elimination.
These challenges were recognized to arise directly from mechanistic limitations inherent in the two-electron nature of the conventional process. Pd catalysis proceeds via two-electron transfer, while Ni catalysis proceeds via single-electron transfer (SET), and the activation energy for SET is extraordinarily low. The development of an activation mode based on SET chemistry would constitute a more efficient strategy for C(sp2)-C(sp3) bond formation.
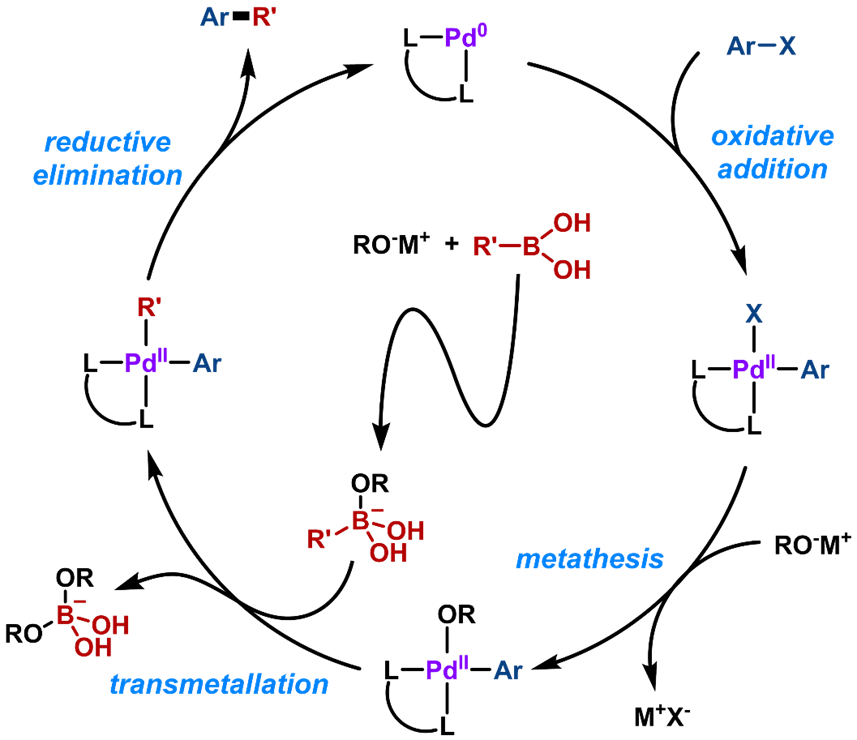
Figure 3. Mechanism of Suzuki Coupling Reaction
2.1 Mechanisms [1]
The mechanism is likely to involve reduction of Ni(II) to Ni (0) complex in the presence of Mn or Zn. The simultaneous oxidative addition of an aryl halide to Ni(0) affords N(II) complexes, which traps the alkyl radicals quickly to form Ni(III) species. The subsequent reductive elimination leads to the desired C(sp2)-C(sp3) bond formation. Finally, the resulting Ni(I) species is proposed to regenerate an alkyl radical to carry the chain (Figure 4). Compared with Pd, Ni tends to be much slower in β-H elimination [2, 3].
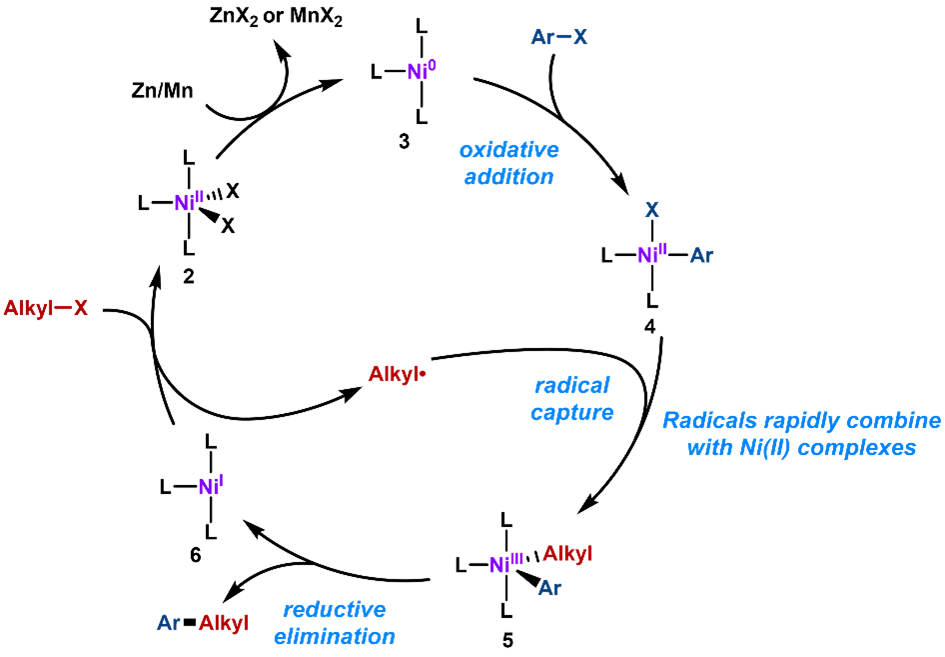
Figure 4. Mechanism for the Nickle-Catalyzed Cross Electrophile Coupling
Given the different mechanisms and reaction conditions, the direct reductive approach offers the opportunity for synthetic orthogonality to conventional approaches.
2.2 Applications
Recent advances in Ni-catalyzed reductive cross-coupling have expanded the “synthetic toolbox” to quickly make more structurally diverse compounds. The commonly used ligands include:
2.2.1 Bipyridines
Bipyridine ligands are used for coupling of aryl halides with alkyl halides, in which one is an iodide and the other can be either an iodide or a bromide (Figure 5). The reaction is tolerant of functional groups that are electrophilic or that have acidic protons, such as ketones, -NHBoc and -NHCbz. And C-B bonds are not reactive under these conditions. The biphosphine ligand dppbz diminished the amount of aryl dimer, and β-H elimination was diminished by the addition of pyridine as a coligand.
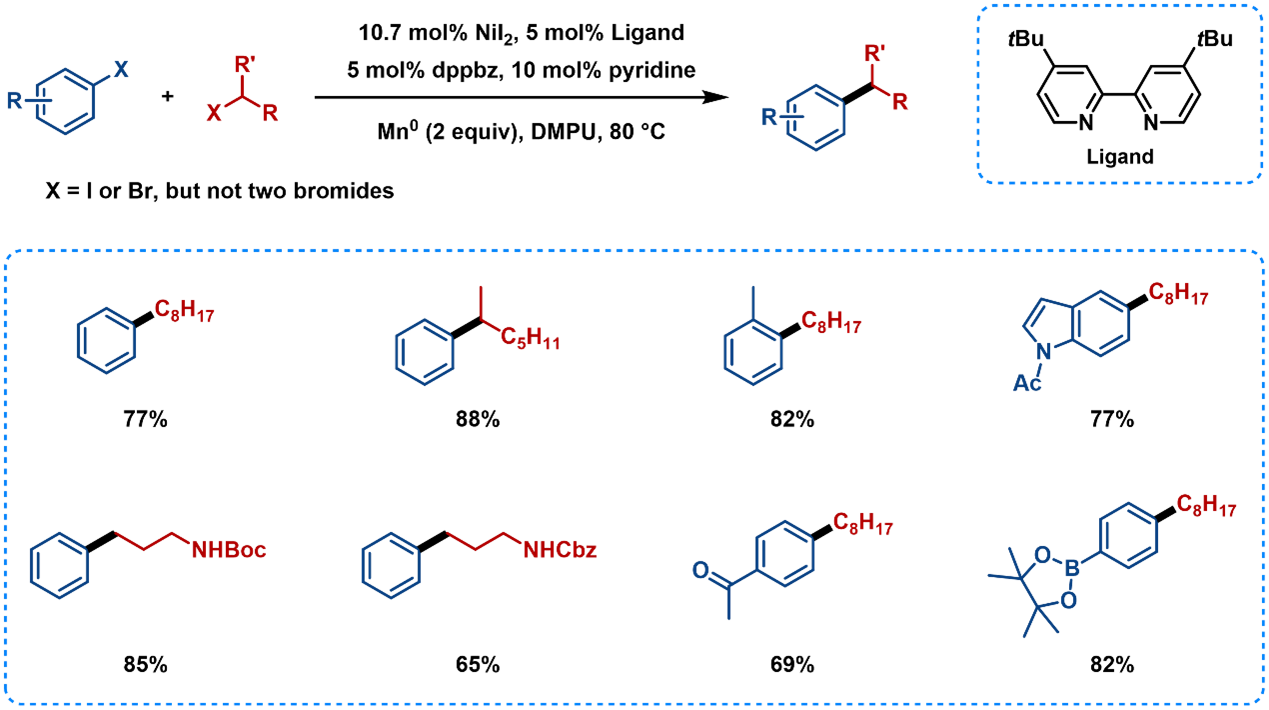
Figure 5. Substrate Scope of the Nickel-Catalyzed Reductive Coupling Using Bipyridine Ligands
2.2.2 Phenanthrolines
A variety of aryl and vinyl bromides are reductively coupled with alkyl bromides in high yields using phenanthroline as a ligand (Figure 6). Functional group tolerance includes ketones, trifluoromethyl, fluoride, hydroxyl, sulfonamide, esters, boronic esters, and organosilicon groups [5]. The addition of NaI may be to help facilitate reduction of the nickel catalyst, generate a small amount of the more reactive alkyl iodide in situ, and promote formation of more reactive nickelate complexes.
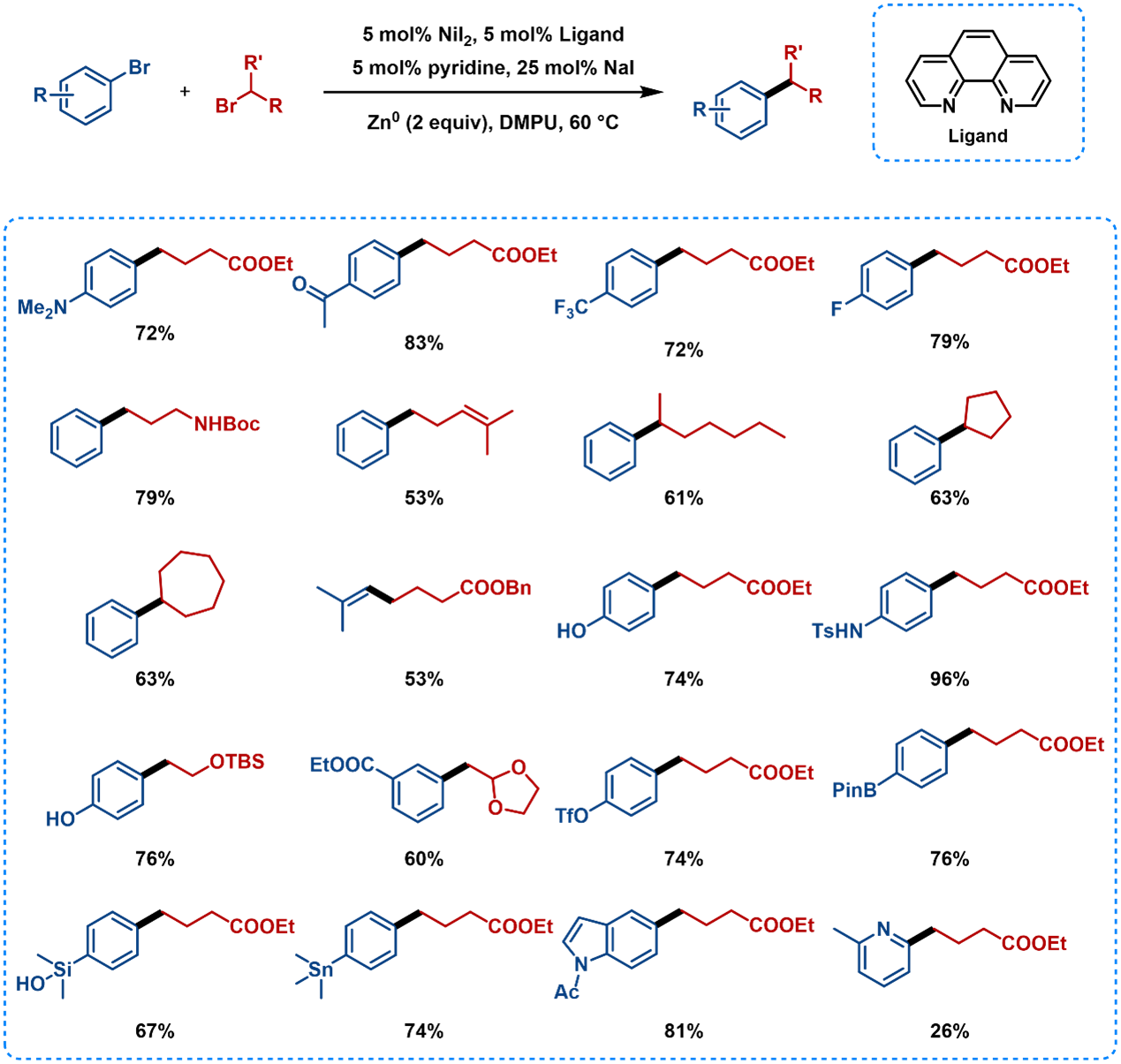
Figure 6. Substrate Scope of Nickel-Catalyzed Reductive Coupling Using Phenanthroline Ligands
2.2.3. Pyridyl Carboxamidines
Pyridyl carboxamidines are highly effective and allow a broad array of heteroaryl halides to be coupled (Figure 7), which are common structures in pharmaceutical chemistry. This reaction also exhibits excellent compatibility with various functional groups, such as amides, boronic acids, and even carboxylic acids. Both primary and secondary alkyl halides can be coupled with 2-, 3-, and 4-pyridyl halides as well as other more complex heterocycles [6,7]. Trifluoroacetic acid is used for activation of zinc.
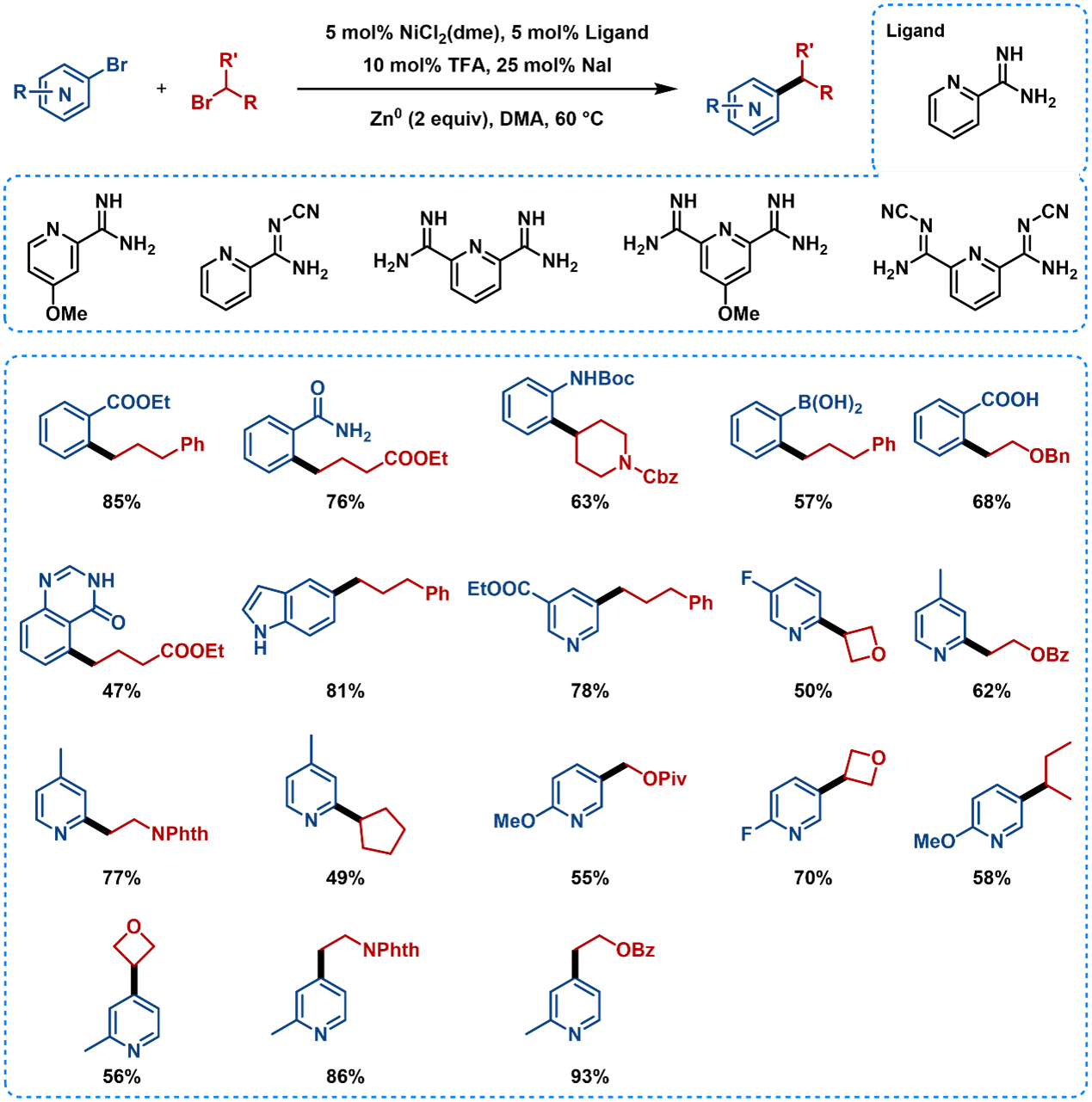
Figure 7. Substrate Scope of Nickel-Catalyzed Reductive Coupling Using Pyridyl Carboxamidine Ligands
These ligands are also used for the coupling of aryl chlorides and alkyl chlorides (Figure 8), which are the most abundant and stable carbon electrophiles. Thiols, phosphate esters, hemiacetals, ketones, ethers, and hydroxyl groups are all well-tolerated [8]. The addition of LiCl may accelerate the reduction of Ni (II) to Ni(0) at the surface of zinc.
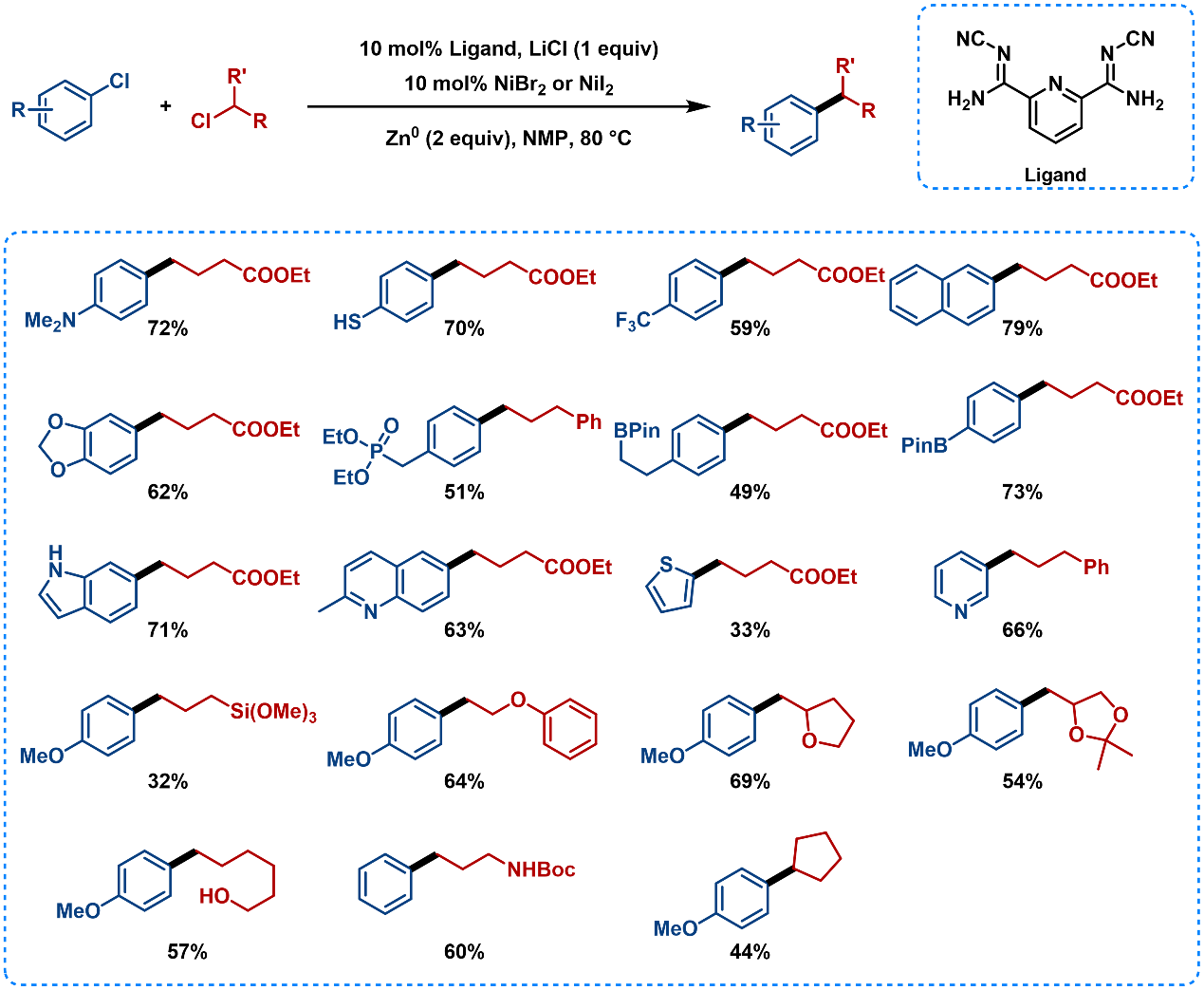
Figure 8. Substrate Scope of Cross-Electrophile Coupling Using Pyridyl Carboxamidine Ligands
2.2.4. Terpyridines
Ni catalyzed C(sp2)−I selective cross-electrophile coupling reaction with tertiary alkyl bromides has been achieved using terpyridine ligands (Figure 9), and the reaction conditions are also suitable for secondary and primary alkyl bromides [9].
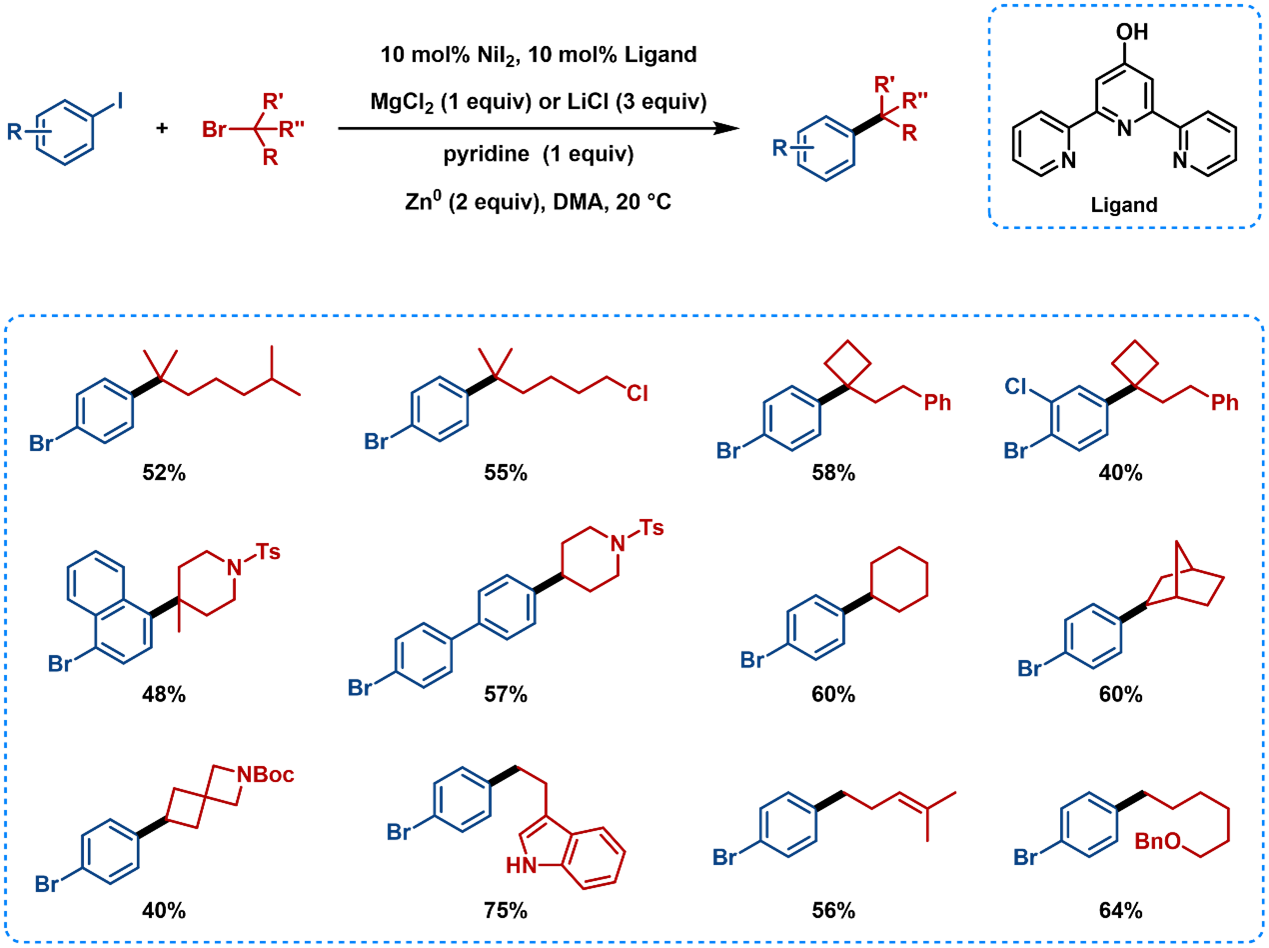
Figure 9. Substrate Scope of Cross-Electrophile Coupling Using Terpyridine Ligands
2.2.5. Di(2-picolyl)amines (DPAs)
DPAs ligands can act as an alternative modular and robust ligand class for the coupling of aryl bromides and alkyl bromides (Figure 10). [10]
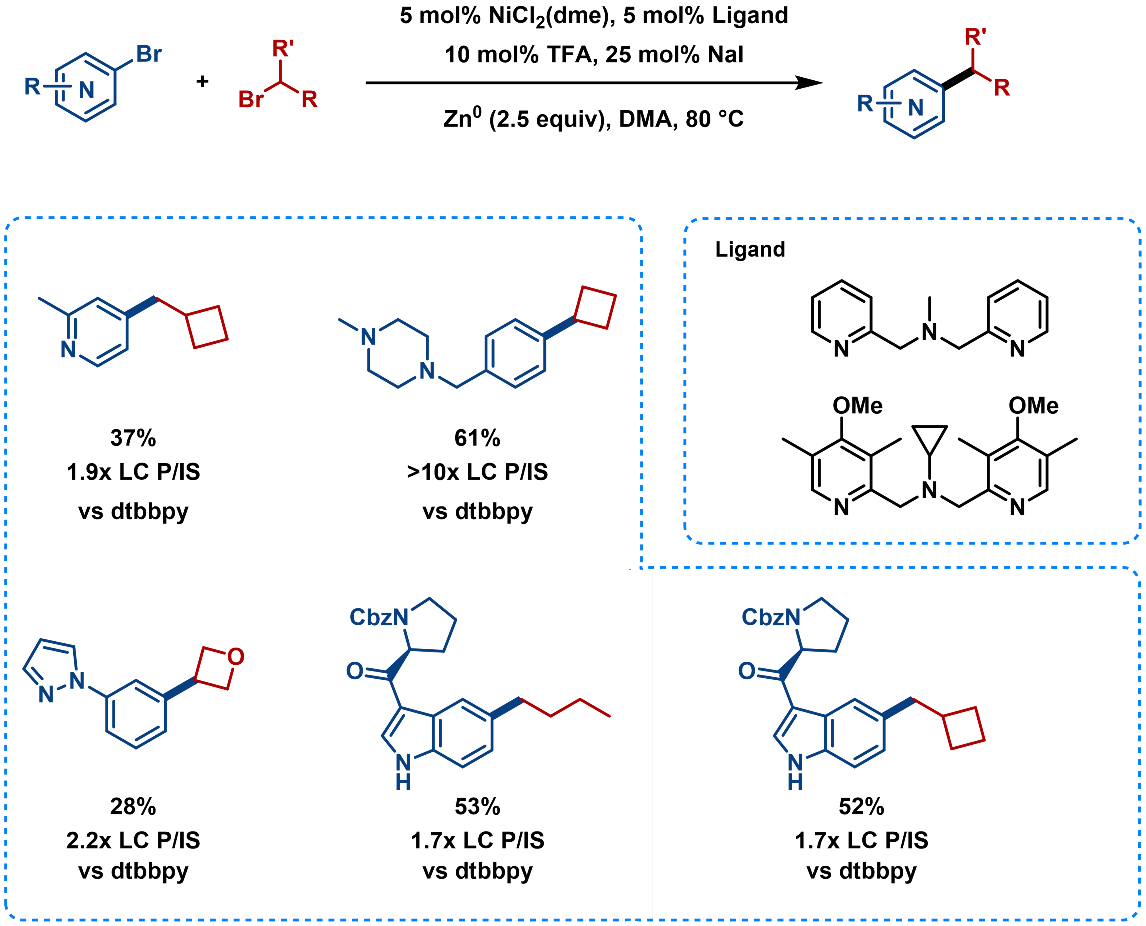
Figure 10. Substrate Scope of Cross-Electrophile Coupling Using DPAs Ligands
2.3 Expanded Application
2.3.1 Alkyl Carboxylic NHP Esters as Alkyl Radical Precursors
Alkyl carboxylic N-hydroxyphthalimide (NHP) esters can also serve as alkyl precursors, facilitating the C(sp2)-C(sp3) bond formation (Figure 11). This method enables the introduction of strained rings, such as cyclopropyl, bicyclo[1.1.1]pentane (BCP), bicyclo[2.1.1]hexane (BCH), and bicyclo[2.2.2]octane. [11] Among these, arylated BCHs was first synthesized via coupling.
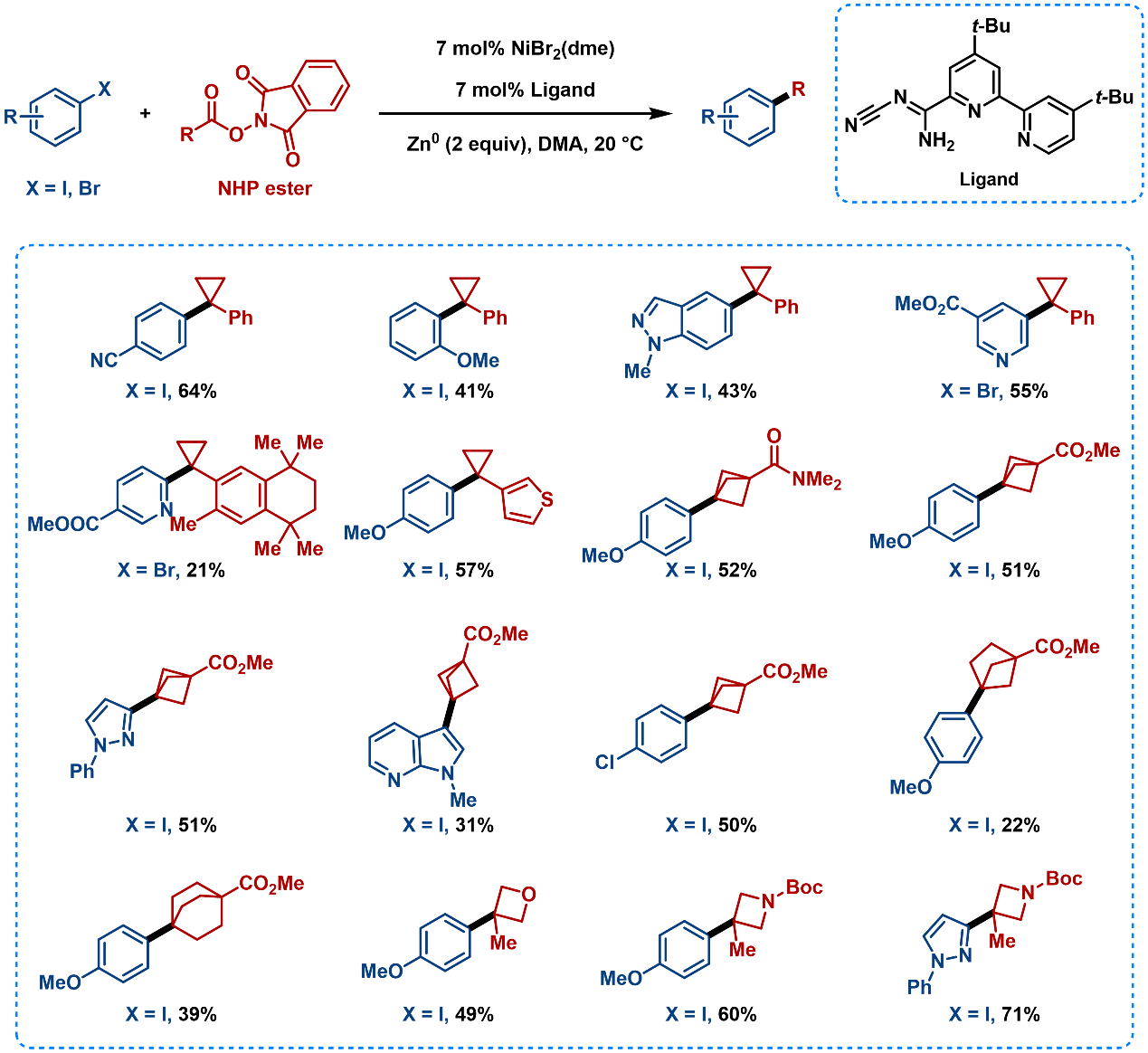
Figure 11. Substrate Scope of Cross-Electrophile Coupling Using Alkyl Carboxylic NHP Esters as Alkyl Radical Precursors
The synthesis of α-arylnitriles via reductive cross-coupling of iodoarenes and alkyl carboxylic NHP esters are accomplished by a combination of trimethylsilyl chloride (TMSCl) and zinc [12]. Direct access to 1-arylcyclopropylamines can be achieved through reductive cross-coupling of cyclopropylamine NHP esters with (hetero)aryl halides [13]. Similarly, using the Boc-protected glycine-derived NHP esters, a series of protected primary benzylamines can be synthesized efficiently [14]. This method further expands the substrate scope.
2.3.2 Bis(Pinacolato)Diboron (B2Pin2) as A Non-Metallic Reductant
B2Pin2 as a non-metallic reductant enables the direct coupling of aryl/vinyl halides with alkyl electrophiles [15]. Alkyl electrophiles ranges from halides, mesylates, to Katritzky salts (Figure 12), with highly functional group tolerance for esters, cyanides, phosphate esters, aldehydes, ketones, boronic esters, sulfonamides and chloride.
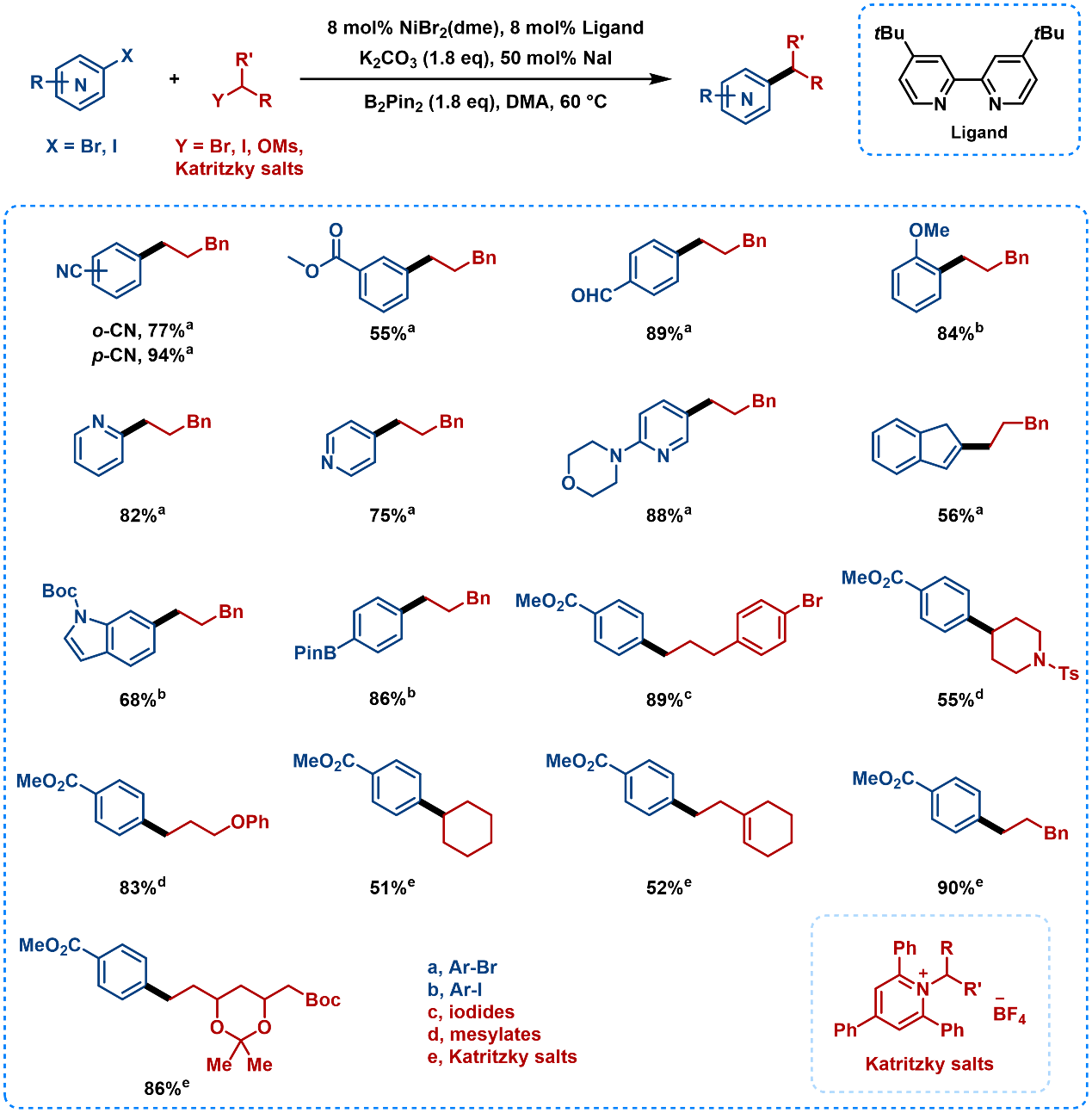
Figure 12. Substrate Scope of Cross-Electrophile Coupling Using B2Pin2 as a Non-Metallic Reductant
This method is also suitable for alcohols [15], which can be converted into alkyl bromides in situ using 2-chloro-3-ethylbenzothiazolium tetrafluoroborate (CEBO) and tetrabutylammonium bromide (TBAB), followed by reductive cross-coupling in one-pot (Figure 13). The use of cost-effective and environment-friendly B2Pin2 further enhances the diversity and versatility of alkyl groups, and simplifies experimental procedures for scalability.
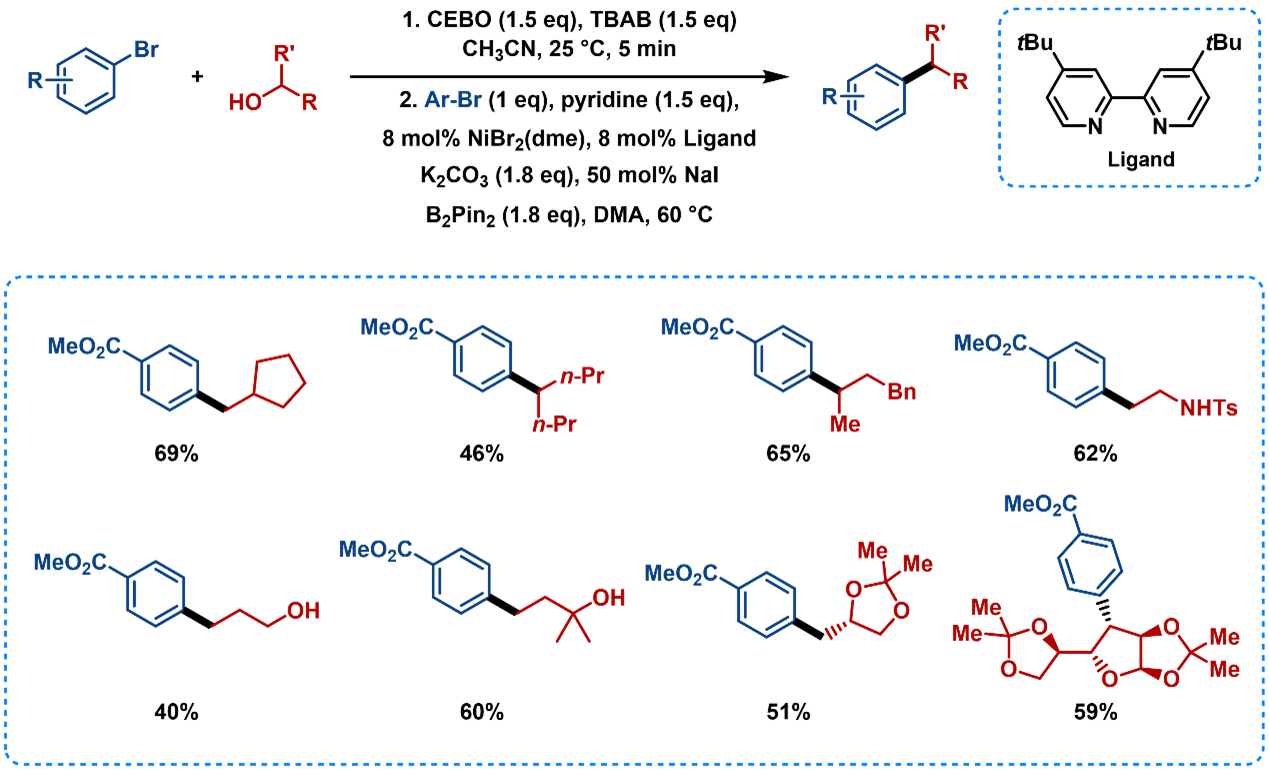
Figure 13. Substrate Scope of Alcohols as Alkyl Radical Precursors
2.4 Advantages
Ni-catalyzed reductive cross-coupling has significant advantages in synthetic chemistry:
- 1. Broad Substrate Generality: Alkyl halides are much more commercially available and more stable than the alkyl nucleophiles.
- 2. Successful Installation of Challenging Group: such as tertiary-alkyl, oxetane, azetidine and bridged rings (Figure 14).
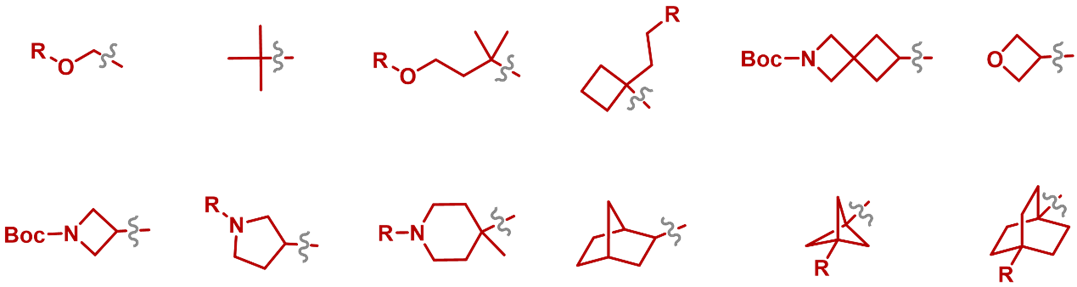
Figure 14. Challenging groups
- 3. Functional Group Compatibility [1, 5]: Since there is no stoichiometric strong bases or nucleophiles, functional group compatibility can be improved, such as -OH, -NHTs, -NHBoc, -NHCbz, -OAc, Ar-OTs, Ar-OTf, -COMe, -CN, -SO₂Me, -SnMe₃, -SiMe₂OH, -BPin, -B(OH)₂, -COOEt, -CONH₂, and -COOH.
- 4. Suitable for Scale-up: This reaction has shown exceptional efficacy ranging from mg-scale to kilogram-scale. Attempts at four strategies for synthesis of key intermediate 7 showed that only the Ni-catalyzed reductive cross-electrophile coupling leads to successful scale-up on a 7 kg scale, with 64% yield and 96.9% purity (Figure 15). [16]
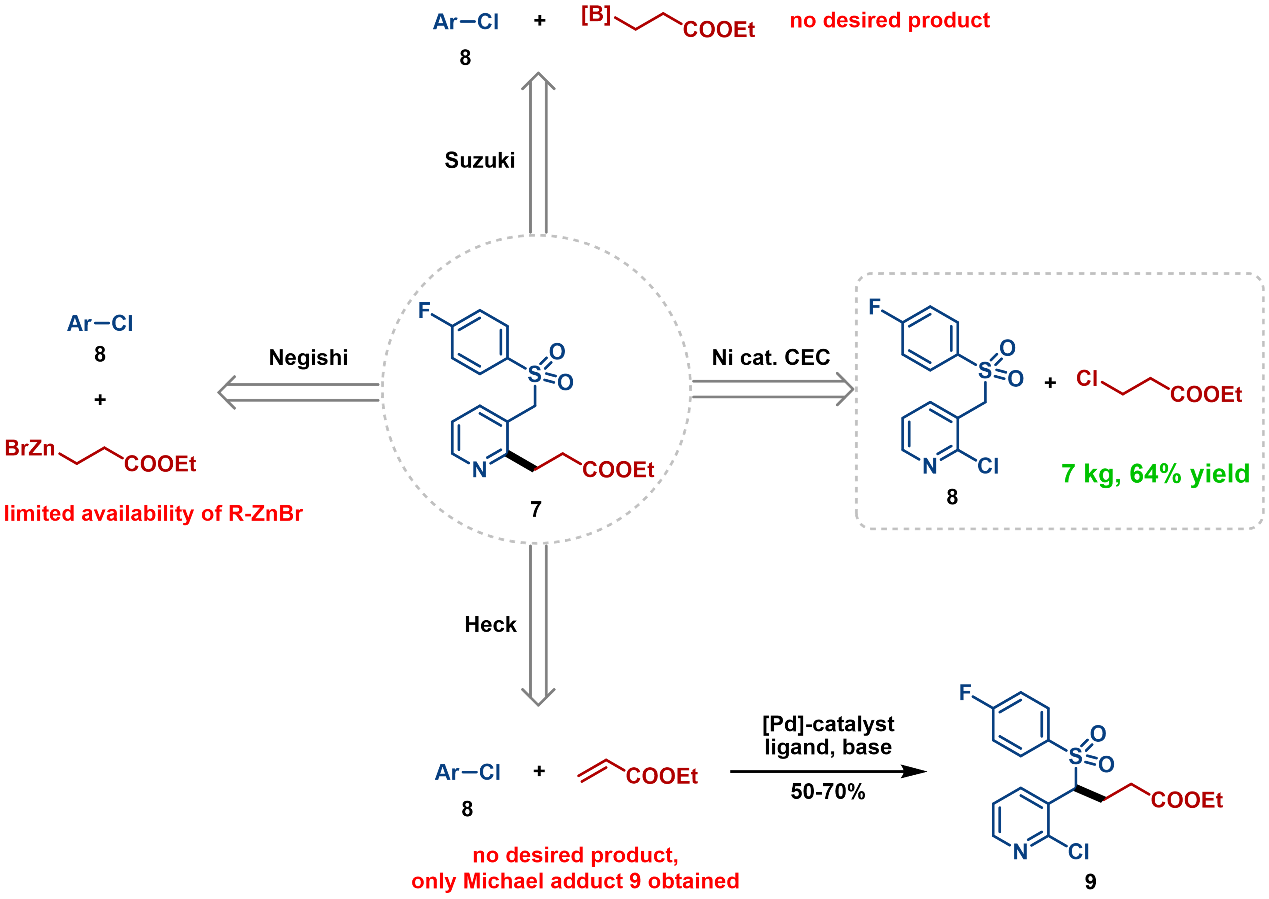
Figure 15. Kilogram-Scale Reaction of Ni-Catalyzed Reduction Coupling
3. Reaction Condition Screening Platform at WuXi AppTec
Given the experimental nature of chemistry, each substrate modification may require fine-tuning of reaction conditions including temperature, solvents, additives, and ligands. Sometimes, side reactions of self-coupling may occur and need to be considered.
WuXi AppTec’s Research Chemistry Services (RCS) has built a Reaction Condition Screening Platform offering tailored screening and optimization services for a wide array of reaction types and substrates. In terms of Ni-catalyzed reductive coupling, the platform enhances drug development efficiency by identifying optimal reaction conditions.
Highlight 1: High Success Rate
The platform has performed numerous Ni-catalyzed reductive coupling reactions for C(sp2)-C(sp3) bond formation. In addition to alkyl halides, alkyl substrates can also be alkyl mesylates or alkyl carboxylic NHP esters. The platform has achieved a 67% success rate in Ni-catalyzed reductive coupling reactions, outperforming conventional methods like Suzuki (43%) and Negishi (31%) coupling.
Highlight 2: Abundant Ligands Library
Catalysts often require specific ligands for optimal catalytic activity. The structure of ligands significantly influences their spatial and electronic attributes, which in turn affects the performance of Ni catalysts. The RCS screening platform has built a compound library with a wealth of diverse ligands (Figure 16).
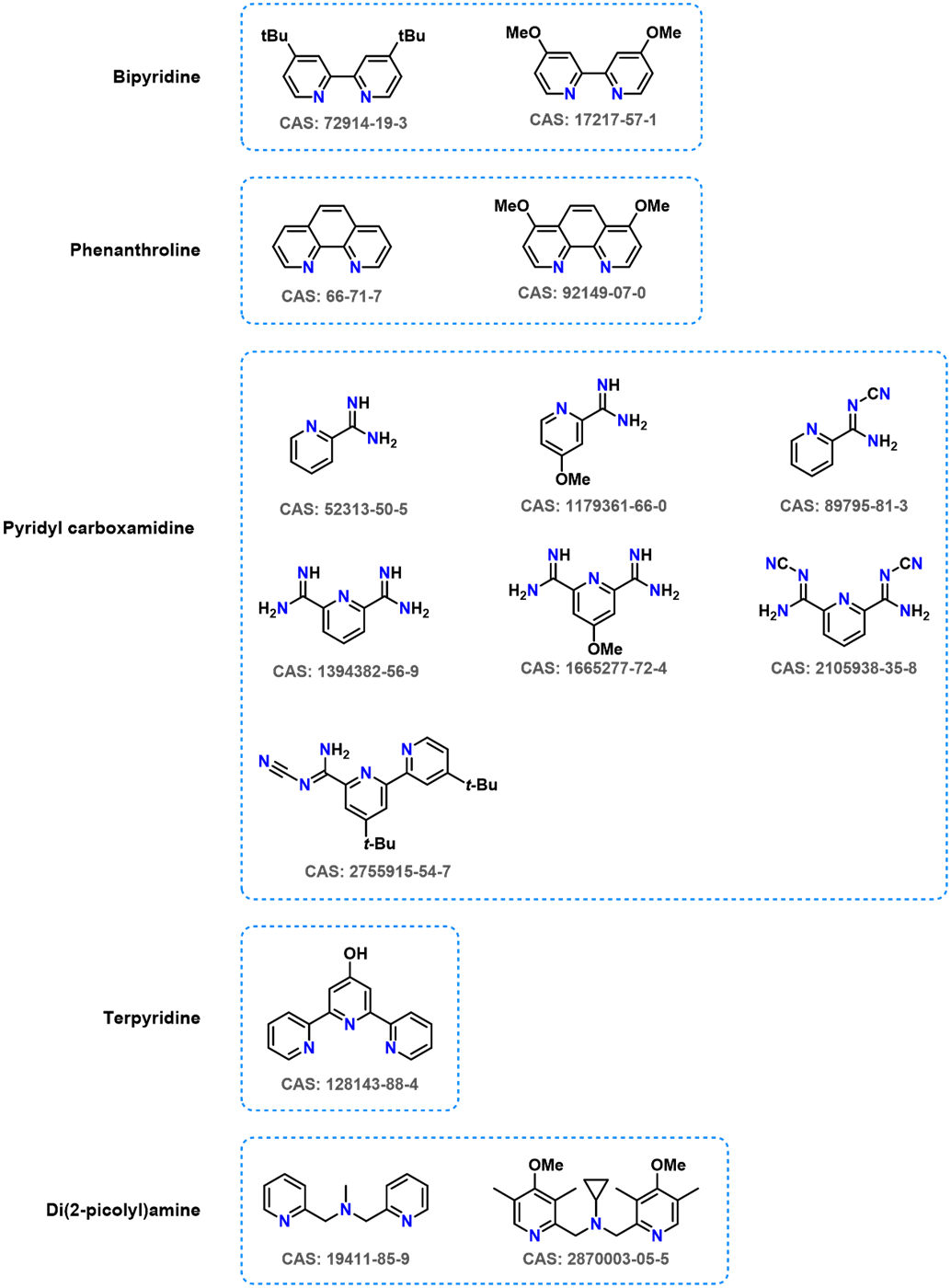
Figure 16. Common Ligands in stock
Highlight 3: Efficient Screening
Advanced facilities and efficient screening techniques are employed to identify the best catalytic systems, ensuring scalability and reproducibility.
Highlight 4: High Reproducibility of Screening Conditions
The screening conditions have high reproducibility, applicable to both small-scale and large-scale, improving efficiency, reducing costs, and ensuring consistent product quality.
4. Case Studies
Making The Unachievable Achievable: Introduction of Challenging Alkyl Groups onto The Aryl Rings
The RCS team successfully introduced challenging alkyl groups that are difficult to access under conventional conditions (Figure 17).
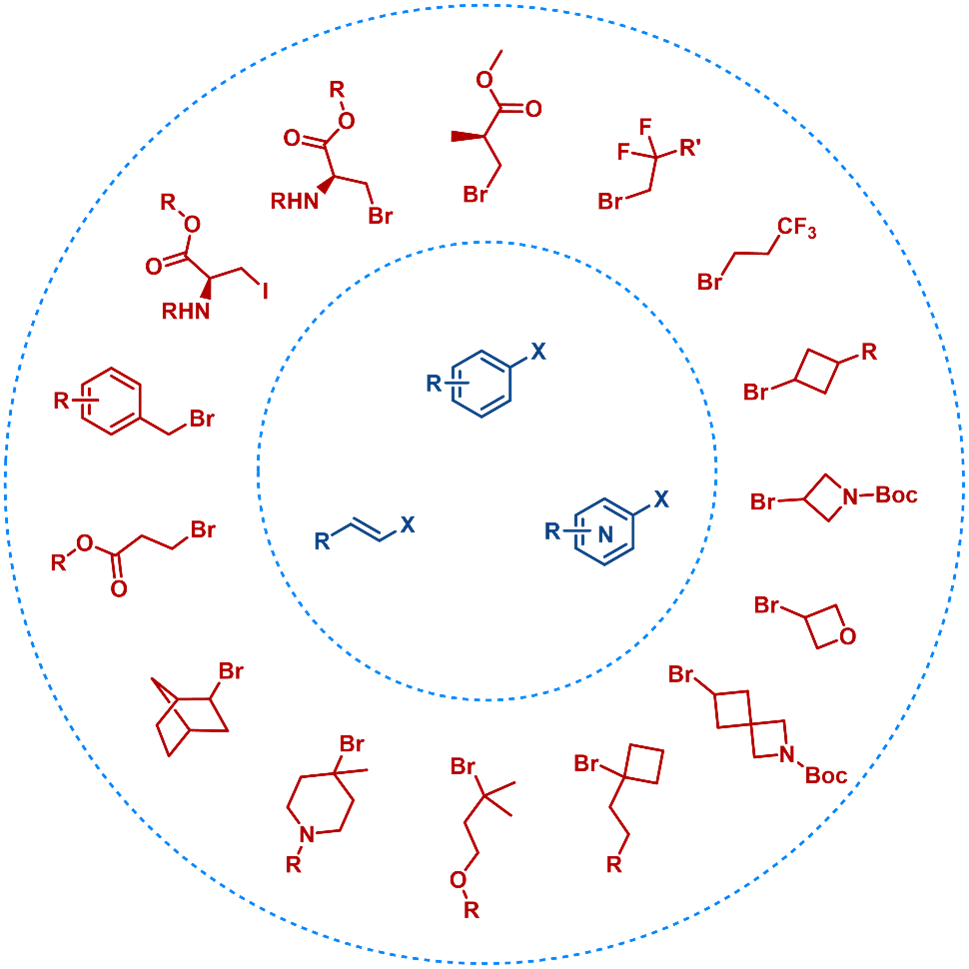
Figure 17. Introduction of Challenging Alkyl Groups onto Aryl Rings
More than “Achievable”, Aim for “Reliable”: Scale-up for coupling of heteroaryl halides with strained rings
Cross-coupling of strained rings remains challenging (Figure 18). The RCS screening platform used Ni-catalyzed reductive coupling method to quickly find the optimal reaction conditions and enabled the successful synthesis of a series of molecules. The isolated yield of these compounds was stable at 40 – 60% yield on a 20 – 30 g scale, demonstrating the method is easily scalable, robust, and reliable.
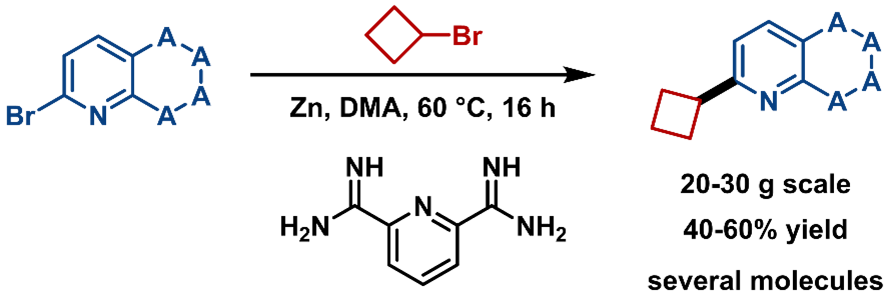
Figure 18. Introduction of Strained Rings onto Heterocycles
By staying current with the latest research and leveraging hands-on experience, the RCS Reaction Condition Screening Platform provides efficient and accurate screening services. This approach accelerates our partners’ drug development processes.
Contact: chemistry_service@wuxiapptec.com
References
- 1. Weix, D. J. Chem. Res. 2015, 48, 1767−1775.
- 2. Tasker, S. Z.; Standley, E. A.; Jamison, T. F. Nature 2014, 509, 299−
- 3. Lin, L. L.; Fu, Y.; Luo, S. W.; Chen, Q.; Guo, Q. X. Organometallics 2004, 23, 2114−2123.
- 4. Everson, D. A.; Shrestha, R.; Weix, D. J. Am. Chem. Soc. 2010, 132, 920−921.
- 5. Everson, D. A.; Jones, B. A.; Weix, D. J. Am. Chem. Soc. 2012, 134, 6146−6159.
- 6. Hansen, E. C.; Pedro, D. J.; Wotal, A. C.; Gower, N. J.; Nelson, J. D.; Caron, S.; Weix, D. J. Nature Chem. 2016, 8, 1126−1130.
- 7. Hansen, E. C.; Li, C.; Yang, S.; Pedro, D. J.; Weix, D. J. Org. Chem. 2017, 82, 7085−7092.
- 8. Kim, S.; Goldfogel, M. J.; Gilbert, M. M.; Weix, D. J. Am. Chem. Soc. 2020, 142, 9902−9907.
- 9. Ying, X.; Li, Y.; Li, L.; Li, C. Chem. Int. Ed. 2023, 62, e202304177.
- 10. Rago, A. J.; Vasilopoulos, A.; Dombrowski, A. W.; Wang, Y. Lett. 2022, 24, 8487−8492.
- 11. Salgueiro, D. C.; Chi, B. K.; Guzei, I. A.; García-Reynaga, P.; Weix, D. J. Chem. Int. Ed. 2022, 61, e202205673.
- 12. Michel, N. W. M.; Gabbey, A. L.; Edjoc, R. K.; Fagbola, E.; Hughes, J. M. E.; Campeau, L. C.; Rousseaux, S. A. L. Org. Chem. 2024 doi: 10.1021/acs.joc.3c02354.
- 13. West, M. S.; Gabbey, A. L.; Huestis, M. P.; Rousseaux, S. A. L. Lett. 2022, 24, 8441−8446.
- 14. Choi, E. S.; Rousseaux, S. A. L.; Huestis, M. P. Synlett 2024, 35, 935−939.
- 15. Sun, D.; Gong, Y.; Wu, Y.; Chen, Y.; Gong, H. Sci. 2024, 11, 2404301.
- 16. Nimmagadda, S. K.; Korapati, S.; Dasgupta, D.; Malik, N. A.; Vinodini, A.; Gangu, A. S.; Kalidindi, S.; Maity, P.; Bondigela, S. S.; Venu, A.; Gallagher, W. P.; Aytar, S.; González-Bobes, F.; Vaidyanathan. R. Process Res. Dev. 2020, 24, 1141−1148.
Disclaimer: This presentation is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds/technologies mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
CAPABILITIES
How can we help?
Get in touch with an expert.