WuXi Biologics
Offering End-to-End Solutions
Chemical Insights | How to Wisely Design Conditions for Buchwald-Hartwig Couplings?
Chemical Insights | How to Wisely Design Conditions for Buchwald-Hartwig Couplings?
Over the past three decades, Pd-catalyzed cross-coupling reactions have become dependable tools of organic synthesis. After the discovery of a variety of biaryl monophosphine ligands, Pd catalyzed C-C, C-X bond formations has become more widely used under mild reaction conditions[1]. Among these cross-coupling reactions, C-N bond formation has a higher demand of condition optimizations due to the multiple types of amine nucleophiles, and some of these are usually challenging. In this article, we will discuss the parameter selection skills for condition optimizations in Buchwald-Hartwig reactions.
A generic mechanism for Buchwald-Hartwig reaction:
The catalytic cycle begins with the reduction of Pd(II) to Pd(0), then the resulting complex undergoes oxidative addition to an aryl (pseudo)halide, followed by the substitution of the amine for the (pseudo) halide and reductive elimination will release desired aryl amination product and at the same time regenerate Pd(0)[2] (Figure 1).
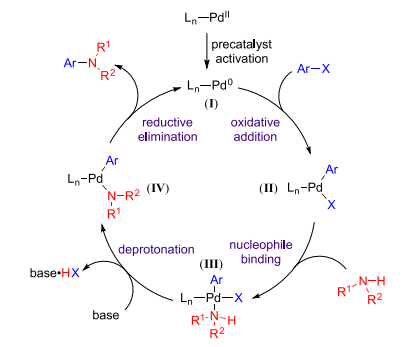
Figure 1 General catalytic cycle for Buchwald-Hartwig reaction[2]
Active catalytic LPd(0) formation:The formation of LPd(0) can be from different
Pd sources. When Pd(II) was used, additional reducing reagents such as amines[3], phosphine ligands[4] and boronic acids[5] are required (Figure 2). The more efficient way is to use Pd pre-catalyst, which will avoid the need for in situ catalyst formation, as only an exposure to base will rapidly generate active catalyst LPd(0) (Figure 3).
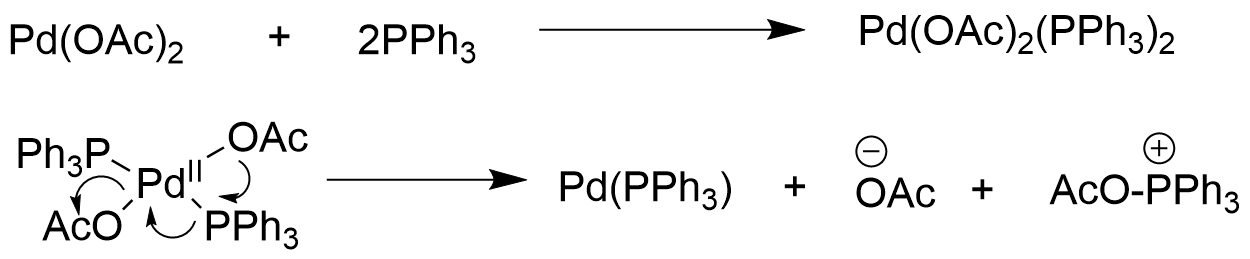
Figure 2 Reduction pathways of Pd(II) to Pd(0)
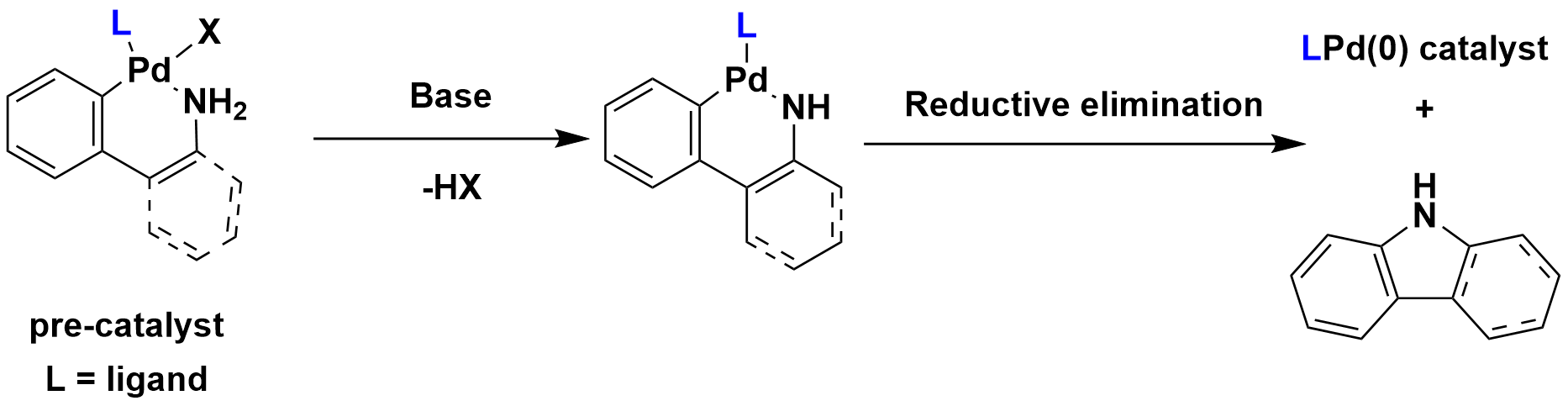
Figure 3 Activation pathways of palladacycle pre-catalysts
Oxidative addition:In terms of the aryl electrophile, the general order of reactivity is ArI > ArBr ~ ArOTf > ArCl ~ ArOMs like in other cross coupling reactions. However, in Buchwald-Hartwig reaction,aryl iodides can be challenging substrates, as the iodide formed during the reaction has been shown to have an inhibitory effect by precipitating the Pd complex and resulting in the off cycle of catalyst [6] (Figure 4). Contrary to conventional wisdom, the reactivity order of aryl electrophiles in Buchwald-Hartwig reaction is as below:
ArBr>ArCl>ArI>ArOTf>ArONf~ArOTs>ArOMs.

Figure 4 Iodide inhibition in Buchwald-Hartwig reaction
Transmetalation: Transmetalation refers to the process of nucleophilic displacement between nucleophiles and (pseudo)halides on the palladium center. It is known to be generally sensitive to steric hindrance around Pd metal. In many cases, the rate of transmetalation is ArOTf>ArCl>ArBr>ArI. When employing aryl triflate as the electrophile, the triflate ion will be dissociated from the metal center, resulting in the Pd complex as cationic and opening a coordination site for the nucleophile, which will make the transmetalation step of the aryl triflates faster [7]. Aryl chlorides are the second fastest electrophiles in the transmetalation step due to the increased polarity of the Pd-Cl bond, and smaller size of Cl relative to Br and I[8].
Reductive elimination: Reductive elimination is the last step of the catalytic cycle and can be facilitated by using electron-poor phosphines or ligands bearing large alkyl substituent such as 1-adamantyl, tert-butyl.
Experienced selection principles of reaction parameters:
Selection of Pd sources
The most commonly employed Pd sources are Pd(OAc)2, PdCl2, PdCl2(MeCN)2, [PdCl(allyl)]2 and Pd2(dba)3, Pd(PPh3)4. The Pd(II) sources need to be reduced to Pd(0) before entering the catalytic cycle, which will lead to a higher loading of catalysts to give an efficient transformation. Since the development of pre-catalysts two decades ago, they have been widely used in routine condition optimizations due to an easy and efficient formation of active catalysts[1] (Figure 5). The features of different Pd sources have been summarized below in Table 1.
| Pre-catalysts | Pd(OAc)2 | Pd2dba3 |
|
|
|
Table 1 Features of different Pd sources
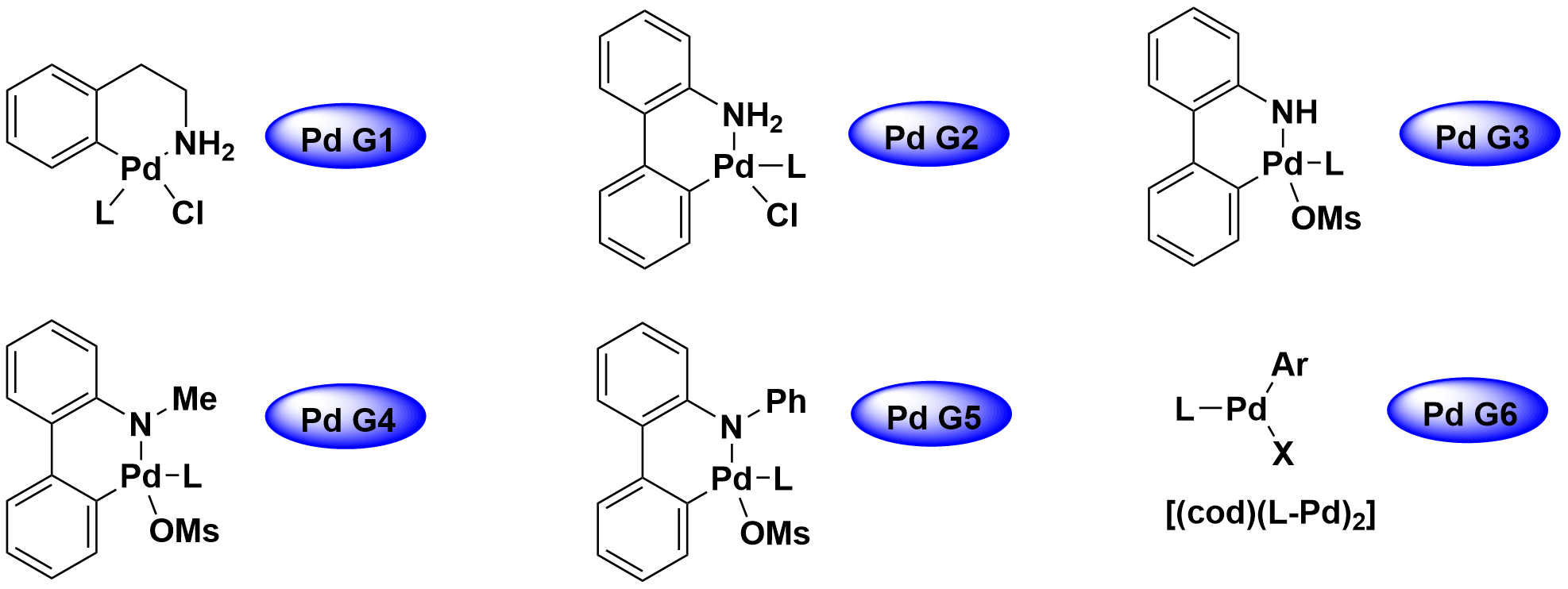
Figure 5 Evolution of Pd ligated Pre-catalysts
G1: Requires activation with strong bases (such as NaOtBu) at room temperature, or weak bases (such as carbonate bases) at 60oC
G2: Could be activated with weak bases (such as carbonate or phosphate bases) at room temperature
G3: By replacing the chloride ligands in 1st and 2nd G-pre with non-coordinating and more electron-poor OMs, 3rd G-pre exhibited the broadest ligand scope
G4: In some rare cases, the carbazole leaving group can inhibit the
catalyst in a reaction, G4-pre solved these problems
G5: Prepared to address carbazole inhibition issues, but caused other issues, as catalyst complexes are difficult to form with bulky ligands
G6: COD-based pre-catalysts are extremely air sensitive; oxidative addition complex based G6 pre-catalysts are air stable and allow better accommodation of bulky ligands
Selection of bases
The most widely used bases for Buchwald-Hartwig couplings are strong bases such as NaOtBu (pKaH=19), and LHMDS, which sometimes will lead to decomposition of substrates carrying base sensitive functional groups. The use of weak bases such as Cs2CO3, K3PO4, K2CO3, NaOPh, NaOTMS allows for broader functional group tolerance. Cs2CO3 (pKaH = 10) is usually included in the condition optimization due to its good solubility in solvents. NaOTMS (pKaH=11) in combination with Gphos Pd G6 provides an efficient transformation of 5-membered ring heteroarenes in C-N bond formation[9]. The deprotonation step is supposed to occur at the solid-liquid boundary which gives a situation that the particle size and the shape of the inorganic bases will have a determined impact on the reaction outcome. So, in the scale up reactions, adding celite to the reaction or grinding bases before use will benefit the reaction through preventing clumping of the inorganic bases. The rate of agitation can also severely impact the rate of these aminations as the high density of inorganic bases will sink to the bottom of the reaction vessel[10]. DBU, P2Et are common organic bases which have good solubility in solvents while less efficient in transmetalation. The combination of organic bases with inorganic bases like DBU+NaTFA is a good solution to base sensitive substrates like amides nucleophiles in the Buchwald-Hartwig couplings. In microwave reactions, organic bases such as DBU, MTBD are always used as the first choices[2].
Selection of temperatures
Typical reaction temperatures used for the coupling is between 80~100oC. When employing weak bases, the reaction temperature required is usually higher in combination with higher catalyst loadings. In many cases the temperature can be decreased to ~40oC. For instance, in the coupling of 5 membered ring heteroarenes, the combination of Gphos Pd G6 with NaOTMS in THF can provide a fast transformation at 40oC in 2 hours[9]. The Buchwald-Hartwig couplings can also be run at room temperature after screening condition parameters in order to maintain good retention of chiral configurations.
Selection of solvents
Insolubility is one of the most common reasons that reactions fail to give good yields of products and is always less estimated. A variety of solvents have been reported for Buchwald-Hartwig reactions, including ethereal solvents (dioxane, THF, CPME, Bu2O, DME),alcohol solvents (n-BuOH, t-AmOH) and aromatic solvents (Toluene, CF3Toluene)[11]. Using of mix solvents with the consideration of solubility and heating temperature is also well developed in condition optimizations. However, chlorinated solvents (e.g., chloroform) and acetonitrile or pyridine have been reported to inhibit the reaction by binding to palladium and should be avoided for using in the reaction[11].
Selection of Ligands
In Figure 6, we summarized the most often employed ligands in Buchwald-Hartwig coupling. The appropriate ligand or pre-catalyst for a given reaction is determined largely by the class of nucleophiles. In Buchwald-Hartwig couplings,the class of nucleophiles can be roughly summarized as aliphatic amines, bulky amines, aryl amines, heteroaryl amines and amides. In the subclass of aliphatic amines, cyclic ones have better performance than linear ones, and primary ones are better than secondary ones. Aryl amines usually have good success rates in the transformation, except diaryl amines. Heteroaryl amines are challenging and will need reaction optimizations to achieve good yields. Primary amides will provide desired arylation products with weak bases, while the reactivity of secondary amides drop a lot. We have summarized the general reactivity trend of different nucleophiles in Figure 7 and ligands or pre-catalysts selection rules for different classes of nucleophiles based on our experimental experience (Figure 8). The final reaction outcome is determined by comprehensive condition parameters and a reference search is always necessary in the design of condition optimizations[1].
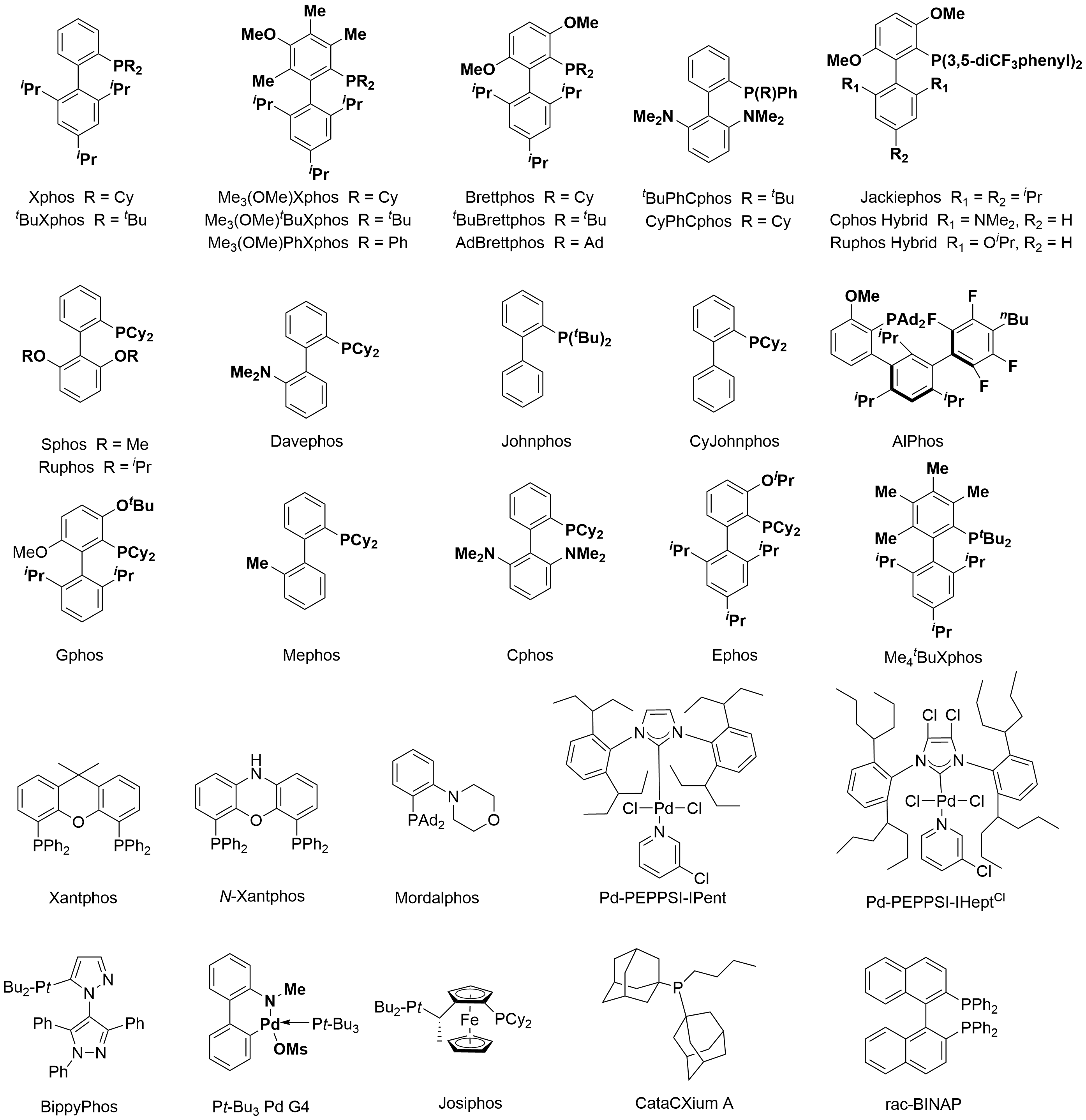
Figure 6 Examples of ligands in Buchwald-Hartwig couplings
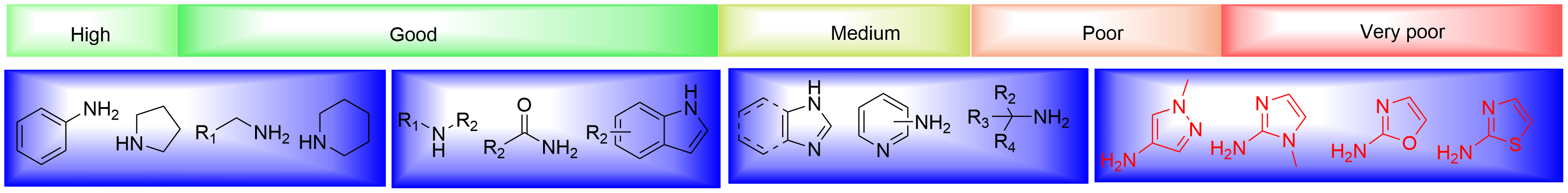
Figure 7 Experience-based reactivity trend of nucleophiles in Buchwald-Hartwig couplings
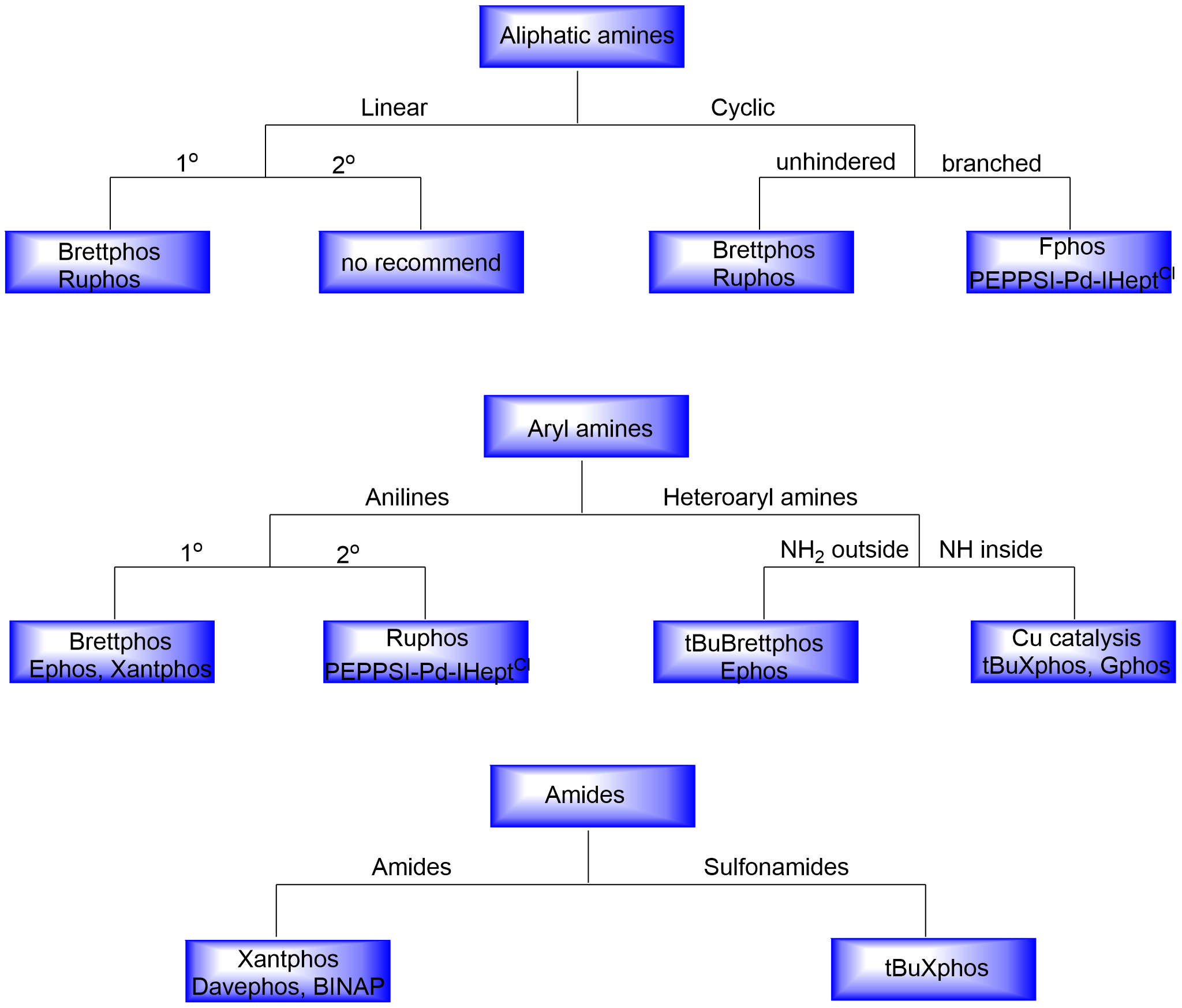
Figure 8 First choice of ligands/pre-catalysts for different nucleophiles in Buchwald-Hartwig couplings
Summary
WuXi AppTec’s Research Chemistry Services (RCS) has built a Reaction Condition Screening Platform offering wise design of condition optimization services for a wide array of reaction types and substrates. In terms of metal catalyzed cross-couplings, the platform enhances drug development efficiency by identifying optimal reaction conditions.
For further questions and requests,
please contact: chemistry_service@wuxiapptec.com
References
[1]) Acc. Chem. Res. 2008, 41, 1461-1473.; Acc. Chem. Res. 2008, 41, 1534-1544.
[2]) Tetrahedron 2019, 75, 4199-4211.
[3]) Chem. Int. Ed. 1996, 35, 2359-2361.
[4]) Organometallics 1992, 11, 3009-3013.
[5]) Am. Chem. Soc. 2003, 125, 6653-6655.
[6]) Am. Chem. Soc. 2009, 131, 5766-5768.
[7]) Organometallics 1995, 14, 1810-1817.
[8]) Am. Chem. Soc. 2010, 132, 14073-14075.
[9]) Am. Chem. Soc. 2023, 145, 3323-3329.
[10]) Proc. Res. Dev. 2006, 10, 472-480.
[11]) Sci. 2011, 2, 27-50.
Disclaimer: This presentation is solely for discussion and informational purposes. It does not constitute an offer to provide the compounds/technologies mentioned. Any order placed will be subject to a thorough IP risk assessment. We will only accept orders for synthesis services if it is determined that no third-party intellectual property rights are infringed.
CAPABILITIES
How can we help?
Get in touch with an expert.